A systematic analysis on the clinical safety and efficacy of onco-virotherapy
- PMID: 34761104
- PMCID: PMC8551473
- DOI: 10.1016/j.omto.2021.09.008
A systematic analysis on the clinical safety and efficacy of onco-virotherapy
Abstract
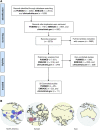
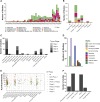
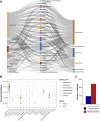
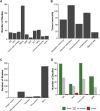
Several onco-virotherapy candidates have been developed and clinically evaluated for the treatment of cancer, and several are approved for clinical use. In this systematic review we explored the clinical impact of onco-virotherapy compared to other cancer therapies by analyzing factors such as trial design, patient background, therapy design, delivery strategies, and study outcomes. For this purpose, we retrieved clinical studies from three platforms: ClinicalTrials.gov, PubMed, and EMBASE. We found that most studies were performed in patients with advanced and metastatic tumors, using a broad range of genetically engineered vectors and mainly administered intratumorally. Therapeutic safety was the most frequently assessed outcome, while relatively few studies focused on immunological antitumor responses. Moreover, only 59 out of 896 clinical studies were randomized controlled trials reporting comparative data. This systemic review thus reveals the need of more, and better controlled, clinical studies to increase our understanding on the application of onco-virotherapy either as a single treatment or in combination with other cancer immunotherapies.
Keywords: cancer; clinical study; immune response; oncolytic virotherapy; outcomes; systematic review; trial.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Rainov N.G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther. 2000;11:2389–2401. - PubMed
-
- Xiao S.W., Xu Y.-Z., Xiao B.-F., Jiang J., Liu C.-Q., Fang Z.W., Li D.-M., Li X.F., Cai Y., Li Y.H. Recombinant adenovirus-p53 gene therapy for advanced unresectable soft-tissue sarcomas. Hum. Gene Ther. 2018;29:699–707. - PubMed
LinkOut - more resources
Full Text Sources

