Chandipura virus induces cell death in cancer cell lines of human origin and promotes tumor regression in vivo
- PMID: 34761105
- PMCID: PMC8554107
- DOI: 10.1016/j.omto.2021.09.009
Chandipura virus induces cell death in cancer cell lines of human origin and promotes tumor regression in vivo
Abstract
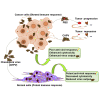
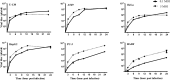
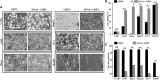
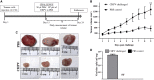
Chandipura virus (CHPV) is an emerging human pathogen of great clinical significance. In this study, we have investigated the susceptibility pattern of both normal and cancer cell lines of human origin to wild-type (wt) CHPV in order to explore the possibility of developing CHPV as an oncolytic vector (OV). Marked cytopathic effect along with enhanced virus output was observed in cancer cell lines (HeLa, A549, U-138, PC-3, and HepG2) in comparison to normal human adult dermal fibroblast (HADF) cells. At an MOI of 0.1, cancer cell lines were differentially susceptible to CHPV, with cells like HeLa and U-138 having pronounced cell death, while the PC-3 were comparatively resistant. All cell lines used in the study except U-138 restricted CHPV infection to varying degrees with IFN-β pre-treatment and supplementation of interferon (IFN) could neither activate the IFN signaling pathway in U-138 cells. Finally, U-138 tumor xenografts established in non-obese diabetic severe combined immunodeficiency (NOD/SCID) mice showed significant delay in tumor growth in the CHPV-challenged animals. Thus, targeted cytopathic effect in cancer cells at a very low dose with restricted replication in normal cells offers a rationale to exploit CHPV as an oncolytic vector in the future.
Keywords: Chandipura virus; U-138 glioma cells; anti-viral response; cytopathic property; interferon-β; oncolytic vector; tumor growth and regression; virus induced cytotoxicity; xenograft model.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Dwibedi B., Sabat J., Hazra R.K., Kumar A., Dinesh D.S., Kar S.K. Chandipura virus infection causing encephalitis in a tribal population of Odisha in eastern India. Natl. Med. J. India. 2015;28:185–187. - PubMed
-
- Gurav Y.K., Tandale B.V., Jadi R.S., Gunjikar R.S., Tikute S.S., Jamgaonkar A.V., Khadse R.K., Jalgaonkar S.V., Arankalle V.A., Mishra A.C. Chandipura virus encephalitis outbreak among children in Nagpur division, Maharashtra, 2007. Indian J. Med. Res. 2010;132:395–399. - PubMed
-
- Chadha M.S., Arankalle V.A., Jadi R.S., Joshi M.V., Thakare J.P., Mahadev P.V., Mishra A.C. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am. J. Trop. Med. Hyg. 2005;73:566–570. - PubMed
LinkOut - more resources
Full Text Sources

