Recovery of platelet-rich red blood cells and acquisition of convalescent plasma with a novel gravity-driven blood separation device
- PMID: 34761451
- PMCID: PMC9298860
- DOI: 10.1111/tme.12830
Recovery of platelet-rich red blood cells and acquisition of convalescent plasma with a novel gravity-driven blood separation device
Abstract
Objectives: Our objectives were to determine the separation characteristics and blood product quality of a gravity-driven microfiltration blood separation system (HemoClear, The Netherlands).
Background: A range of centrifugal blood separation devices, including intraoperative cell salvage devices (cell savers) and apheresis machines, are available to assist in preparing both allogenic and autologous blood products. These devices are expensive to operate and require extensive training.
Methods and materials: Nine whole blood units were collected under standard conditions and analysed for haematological parameters, thromboelastographic properties, platelet morphology and activation, and red blood cell (RBC) deformability and morphology. Three whole blood units were separated by means of the HemoClear device, into a liquid and cellular component. The cellular component was diluted with SAGM and cold stored for 14 days. To simulate cell salvage six whole blood units were diluted with isotonic saline, followed by multiple HemoClear separation rounds.
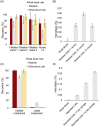
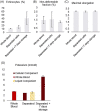
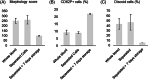
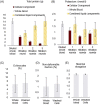
Results: The recovery of both RBCs (100 ± 1.6%) and white blood cells (99 ± 4.5%) after undiluted filtration were very high, while platelet recovery was high (83 ± 3.0%). During the filtration, and cold storage after filtration storage both the non-deformable RBC fraction and the RBC maximum elongation remained stable. Parameters of thromboelastography indicated that platelets remain functional after filtration and after 7 days of cold storage. In the cell salvage simulation the total protein load in the cellular fraction was reduced by 65 ± 4.1% after one washing round and 84 ± 1.9% after two consecutive washing rounds.
Conclusion: The novel blood filter studied effectively separates whole blood into diluted plasma and platelet-rich RBCs. Moreover, the device effectively washed diluted whole blood, driving over 80% of proteins to the liquid component.
Keywords: autologous blood; autologous blood technology; blood filter; blood separation; cell salvage; cell salvage technology; convalescent plasma; platelet-rich RBC.
© 2021 The Authors. Transfusion Medicine published by John Wiley & Sons Ltd on behalf of British Blood Transfusion Society.
Conflict of interest statement
Arno Nierich is the inventor of the HemoClear device, holds patent right to the device's technology and owns shares in HemoClear BV. Dion Osemwengie is employed by HemoClear BV.
Figures

Similar articles
-
Comparison of Washing Efficiency and Recovery of Blood Cells Between Centrifugation, Coarse Filtration and Microfiltration Techniques to Prepare Autologous Blood for Transfusion.J Blood Med. 2022 Sep 30;13:549-558. doi: 10.2147/JBM.S367918. eCollection 2022. J Blood Med. 2022. PMID: 36204560 Free PMC article.
-
An In Vitro Pilot Study Comparing the Novel HemoClear Gravity-Driven Microfiltration Cell Salvage System with the Conventional Centrifugal XTRA™ Autotransfusion Device.Anesthesiol Res Pract. 2020 Sep 8;2020:9584186. doi: 10.1155/2020/9584186. eCollection 2020. Anesthesiol Res Pract. 2020. PMID: 32963523 Free PMC article.
-
Evaluation of canine red blood cell quality after processing with an automated cell salvage device.J Vet Emerg Crit Care (San Antonio). 2016 May;26(3):373-83. doi: 10.1111/vec.12472. Epub 2016 Apr 14. J Vet Emerg Crit Care (San Antonio). 2016. PMID: 27078857
-
Bacteria-induced release of white cell--and platelet-derived vascular endothelial growth factor in vitro.Vox Sang. 2001 Apr;80(3):170-8. doi: 10.1046/j.1423-0410.2001.00028.x. Vox Sang. 2001. PMID: 11449957
-
Tangential flow filtration facilitated washing of human red blood cells: A proof-of-concept study.Vox Sang. 2022 Jun;117(6):803-811. doi: 10.1111/vox.13259. Epub 2022 Mar 9. Vox Sang. 2022. PMID: 35262216 Free PMC article.
Cited by
-
Reduced Mortality among COVID-19 ICU Patients after Treatment with HemoClear Convalescent Plasma in Suriname.mBio. 2023 Apr 25;14(2):e0337922. doi: 10.1128/mbio.03379-22. Epub 2023 Feb 23. mBio. 2023. PMID: 36815780 Free PMC article.
-
Therapeutic plasma exchange in critically ill patients in low-income and lower-middle-income countries: medical need and feasibility.J Glob Health. 2025 Jul 25;15:04214. doi: 10.7189/jogh.15.04214. J Glob Health. 2025. PMID: 40709581 Free PMC article.
-
Comparison of Washing Efficiency and Recovery of Blood Cells Between Centrifugation, Coarse Filtration and Microfiltration Techniques to Prepare Autologous Blood for Transfusion.J Blood Med. 2022 Sep 30;13:549-558. doi: 10.2147/JBM.S367918. eCollection 2022. J Blood Med. 2022. PMID: 36204560 Free PMC article.
References
-
- Schmidt A, Refaai M, Kirkley S & Blumberg N Proven and Potential Clinical Benefits of Washing Red Blood Cells before Transfusion: Current Perspectives. Int J Clin Transfus Med. 2016;4:79–88.
-
- Global status report on blood safety and availability 2016 . Licence: CC BY‐NC‐SA 3.0 IGO. World Health Organization; 2017.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

