Different molecular changes underlie the same phenotypic transition: Origins and consequences of independent shifts to homostyly within species
- PMID: 34761469
- PMCID: PMC10078681
- DOI: 10.1111/mec.16270
Different molecular changes underlie the same phenotypic transition: Origins and consequences of independent shifts to homostyly within species
Abstract
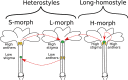
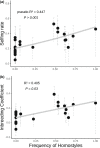
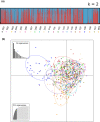
The repeated transition from outcrossing to selfing is a key topic in evolutionary biology. However, the molecular basis of such shifts has been rarely examined due to lack of knowledge of the genes controlling these transitions. A classic example of mating system transition is the repeated shift from heterostyly to homostyly. Occurring in 28 angiosperm families, heterostyly is characterized by the reciprocal position of male and female sexual organs in two (or three) distinct, usually self-incompatible floral morphs. Conversely, homostyly is characterized by a single, self-compatible floral morph with reduced separation of male and female organs, facilitating selfing. Here, we investigate the origins of homostyly in Primula vulgaris and its microevolutionary consequences by integrating surveys of the frequency of homostyles in natural populations, DNA sequence analyses of the gene controlling the position of female sexual organs (CYPᵀ), and microsatellite genotyping of both progeny arrays and natural populations characterized by varying frequencies of homostyles. As expected, we found that homostyles displace short-styled individuals, but long-style morphs are maintained at low frequencies within populations. We also demonstrated that homostyles repeatedly evolved from short-styled individuals in association with different types of loss-of-function mutations in CYPᵀ. Additionally, homostyly triggers a shift to selfing, promoting increased inbreeding within and genetic differentiation among populations. Our results elucidate the causes and consequences of repeated transitions to homostyly within species, and the putative mechanisms precluding its fixation in P. vulgaris. This study represents a benchmark for future analyses of losses of heterostyly in other angiosperms.
Keywords: Primula; heterostyly; intraspecific; loss-of-function mutations; mating system; selfing.
© 2021 The Authors. Molecular Ecology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Comment in
-
Primrose homostyles: A classic case of possible balancing selection revisited.Mol Ecol. 2023 Jan;32(1):30-32. doi: 10.1111/mec.16746. Epub 2022 Nov 6. Mol Ecol. 2023. PMID: 36271781 Free PMC article.
References
-
- Barmentlo, S. H. , Meirmans, P. G. , Luijten, S. H. , Triest, L. , & Oostermeijer, J. G. B. (2017). Outbreeding depression and breeding system evolution in small, remnant populations of Primula vulgaris: consequences for genetic rescue. Conservation Genetics, 19, 545–554. 10.1007/s10592-017-1031-x - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

