A Multifunctional Neutralizing Antibody-Conjugated Nanoparticle Inhibits and Inactivates SARS-CoV-2
- PMID: 34761549
- PMCID: PMC8646742
- DOI: 10.1002/advs.202103240
A Multifunctional Neutralizing Antibody-Conjugated Nanoparticle Inhibits and Inactivates SARS-CoV-2
Abstract
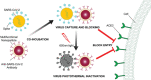
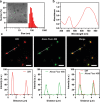
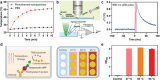
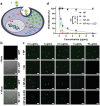
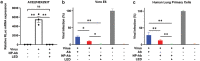
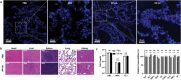
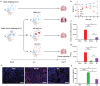
The outbreak of 2019 coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in a global pandemic. Despite intensive research, the current treatment options show limited curative efficacies. Here the authors report a strategy incorporating neutralizing antibodies conjugated to the surface of a photothermal nanoparticle (NP) to capture and inactivate SARS-CoV-2. The NP is comprised of a semiconducting polymer core and a biocompatible polyethylene glycol surface decorated with high-affinity neutralizing antibodies. The multifunctional NP efficiently captures SARS-CoV-2 pseudovirions and completely blocks viral infection to host cells in vitro through the surface neutralizing antibodies. In addition to virus capture and blocking function, the NP also possesses photothermal function to generate heat following irradiation for inactivation of virus. Importantly, the NPs described herein significantly outperform neutralizing antibodies at treating authentic SARS-CoV-2 infection in vivo. This multifunctional NP provides a flexible platform that can be readily adapted to other SARS-CoV-2 antibodies and extended to novel therapeutic proteins, thus it is expected to provide a broad range of protection against original SARS-CoV-2 and its variants.
Keywords: COVID-19; SARS-CoV-2; multifunctional nanoparticle; photothermal therapy; virus inactivation.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
A Neutralizing Antibody-Conjugated Photothermal Nanoparticle Captures and Inactivates SARS-CoV-2.bioRxiv [Preprint]. 2020 Nov 30:2020.11.30.404624. doi: 10.1101/2020.11.30.404624. bioRxiv. 2020. Update in: Adv Sci (Weinh). 2022 Jan;9(2):e2103240. doi: 10.1002/advs.202103240. PMID: 33269351 Free PMC article. Updated. Preprint.
References
-
- Ranney M. L., Griffeth V., Jha A. K., N. Engl. J. Med. 2020, 382, e41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
