The utility of SARS-CoV-2-specific serology in COVID-19 diagnosis
- PMID: 34761846
- PMCID: PMC8652559
- DOI: 10.1111/1753-6405.13155
The utility of SARS-CoV-2-specific serology in COVID-19 diagnosis
Abstract
Introduction: In May 2020, The Communicable Diseases Network of Australia (CDNA) case definition introduced serological criteria to support the diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We present findings that support the utility of SARS-CoV-2-specific serology for public health investigations.
Methods: From 24 January to 31 July 2020, the following information was collected from individuals with positive SARS-CoV-2-specific immunofluorescence antibody tests: history of contact with COVID-19 cases; recent travel; symptoms consistent with COVID-19; and SARS-CoV-2 nucleic acid testing (NAT) results. Individuals were classified as confirmed or probable by CDNA criteria or additionally as possible (SARS-CoV-2-specific IgG positive with compatible symptoms or epidemiologic risk) or indeterminate (SARS-CoV-2-specific IgA/IgM positive only) cases.
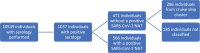
Results: A total of 10,595 individuals were tested in the six-month period. Of these, 9.8% (1,037) individuals had positive SARS-CoV-2-specific serology of which 566 (53.6%) were NAT-confirmed COVID-19 cases and 286 (27.6%) were part of a cruise ship outbreak sero-survey. The remaining 185 individuals (NAT negative) were individually classified as serologically confirmed (4, 0.4%), probable (72, 6.9%) possible (66, 6.4%) and indeterminate (38, 3.7%) cases. Maternal antibody transfer was inferred in one infant and four were unclassified.
Conclusion: SARS-CoV-2-specific serology is a key diagnostic tool for retrospective identification of COVID-19 infection. Implications for public health: SARS-CoV-2 specific serology can enhance the ability to find cases, link missing cases in clusters of infection and identify the epidemiological extent of SARS-CoV-2 outbreaks. A combination of epidemiological criteria, clinical criteria and a quantitative serological test can be used as an adjunct to classify SARS-CoV-2 cases. Our study confirms the low level of community transmission in NSW during the first year of the COVID-19 pandemic.
Keywords: case definitions; covid-19; serology.
© 2021 The Authors.
Figures
References
-
- NSW Ministry of Health . State Government of New South Wales; Sydney (AUST): 2020. COVID‐19 Cases by Date and Likely Source of Infection [Internet]Available from: https://data.nsw.gov.au/data/dataset/nsw-covid-19-cases-... [cited 2020 Nov].
-
- Australian Department of Health . Government of Australia; Canberra (AUST): 2020. Coronavirus (COVID‐19) at a Glance – 1 July 2020 [Internet]Available from: https://www.health.gov.au/sites/default/files/documents/... [cited 2020 Aug].
-
- Australian Department of Health COVID‐19, Australia: Epidemiology report 19 (Fortnightly reporting period ending June 21, 2020) Commun Dis Intell (2018) 2020;44 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous