A Microfluidic Platform for Sequential Assembly and Separation of Synthetic Cell Models
- PMID: 34761904
- PMCID: PMC8609574
- DOI: 10.1021/acssynbio.1c00371
A Microfluidic Platform for Sequential Assembly and Separation of Synthetic Cell Models
Abstract
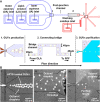
Cell-sized vesicles like giant unilamellar vesicles (GUVs) are established as a promising biomimetic model for studying cellular phenomena in isolation. However, the presence of residual components and byproducts, generated during vesicles preparation and manipulation, severely limits the utility of GUVs in applications like synthetic cells. Therefore, with the rapidly growing field of synthetic biology, there is an emergent demand for techniques that can continuously purify cell-like vesicles from diverse residues, while GUVs are being simultaneously synthesized and manipulated. We have developed a microfluidic platform capable of purifying GUVs through stream bifurcation, where a vesicles suspension is partitioned into three fractions: purified GUVs, residual components, and a washing solution. Using our purification approach, we show that giant vesicles can be separated from various residues─which range in size and chemical composition─with a very high efficiency (e = 0.99), based on size and deformability of the filtered objects. In addition, by incorporating the purification module with a microfluidic-based GUV-formation method, octanol-assisted liposome assembly (OLA), we established an integrated production-purification microfluidic unit that sequentially produces, manipulates, and purifies GUVs. We demonstrate the applicability of the integrated device to synthetic biology through sequentially fusing SUVs with freshly prepared GUVs and separating the fused GUVs from extraneous SUVs and oil droplets at the same time.
Keywords: artificial cell models; bottom-up synthesis; giant unilamellar vesicles; giant vesicle purification; lipid bilayer; microfluidics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

