Assessment of Arsenic Trioxide and All-trans Retinoic Acid for the Treatment of Pediatric Acute Promyelocytic Leukemia: A Report From the Children's Oncology Group AAML1331 Trial
- PMID: 34762093
- PMCID: PMC8587220
- DOI: 10.1001/jamaoncol.2021.5206
Assessment of Arsenic Trioxide and All-trans Retinoic Acid for the Treatment of Pediatric Acute Promyelocytic Leukemia: A Report From the Children's Oncology Group AAML1331 Trial
Abstract
Importance: All-trans retinoic acid (ATRA) and arsenic trioxide therapy without the use of maintenance therapy has been found to be beneficial for the treatment of adults with standard-risk acute promyelocytic leukemia (APL). However, it is unclear whether similar regimens are safe and beneficial for the treatment of high-risk APL or pediatric patients with standard-risk APL.
Objective: To assess whether treatment with an ATRA and arsenic trioxide-based regimen is safe and allows for the elimination or substantial reduction of chemotherapy use among pediatric patients with standard-risk or high-risk APL, respectively.
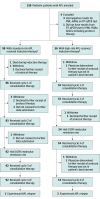
Design, setting, and participants: The Children's Oncology Group AAML1331 study is a nonrandomized, noninferiority trial that examined survival outcomes among 154 pediatric patients with APL compared with a historical control group of patients with APL from the AAML0631 study. Patients aged 1 to 21 years were enrolled at 85 pediatric oncology centers (members of the Children's Oncology Group) in Australia, Canada, and the US from June 29, 2015, to May 7, 2019, with follow-up until October 31, 2020. All patients had newly diagnosed APL and were stratified into standard-risk APL (white blood cell count <10 000/μL) and high-risk APL (white blood cell count ≥10 000/μL) cohorts.
Interventions: All patients received ATRA and arsenic trioxide continuously during induction therapy and intermittently during 4 consolidation cycles. Patients with high-risk APL received 4 doses of idarubicin during induction therapy only. The duration of therapy was approximately 9 months, and no maintenance therapy was administered.
Main outcomes and measures: Event-free survival (EFS) at 2 years after diagnosis.
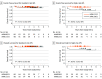
Results: Among 154 patients (median age, 14.4 years [range, 1.1-21.7 years]; 81 male participants [52.6%]) included in the analysis, 98 patients (63.6%) had standard-risk APL, and 56 patients (36.4%) had high-risk APL. The median follow-up duration was 24.7 months (range, 0-49.5 months) for patients with standard-risk APL and 22.8 months (range, 0-47.7 months) for patients with high-risk APL. Patients with standard-risk APL had a 2-year EFS rate of 98.0% and an overall survival rate of 99.0%; adverse events included 1 early death during induction therapy and 1 relapse. Patients with high-risk APL had a 2-year EFS rate of 96.4% and an overall survival rate of 100%; adverse events included 2 relapses and 0 deaths. These outcomes met predefined noninferiority criteria (noninferiority margin of 10% among those with standard-risk APL and 14.5% among those with high-risk APL).
Conclusions and relevance: In this nonrandomized, noninferiority trial, pediatric patients with standard-risk APL who received treatment with a chemotherapy-free ATRA and arsenic trioxide regimen experienced positive outcomes. Patients with high-risk APL also had positive outcomes when treated with a novel ATRA and arsenic trioxide-based regimen that included 4 doses of idarubicin during induction therapy only and no maintenance therapy. The 2-year EFS estimates were noninferior to the historical comparator group, and advantages of the regimen included shorter treatment duration, lower exposure to anthracycline and intrathecal chemotherapy, and fewer days hospitalized.
Trial registration: ClinicalTrials.gov Identifier: NCT02339740.
Conflict of interest statement
Figures
References
-
- Shankar SM, Marina N, Hudson MM, et al. ; Cardiovascular Disease Task Force of the Children’s Oncology Group . Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121(2):e387-e396. doi:10.1542/peds.2007-0575 - DOI - PubMed
-
- Kutny MA, Alonzo TA, Gerbing RB, et al. . Arsenic trioxide consolidation allows anthracycline dose reduction for pediatric patients with acute promyelocytic leukemia: report from the Children’s Oncology Group phase III historically controlled trial AAML0631. J Clin Oncol. 2017;35(26):3021-3029. doi:10.1200/JCO.2016.71.6183 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

