Changes in Prostate-Specific Antigen Testing Relative to the Revised US Preventive Services Task Force Recommendation on Prostate Cancer Screening
- PMID: 34762100
- PMCID: PMC8587214
- DOI: 10.1001/jamaoncol.2021.5143
Changes in Prostate-Specific Antigen Testing Relative to the Revised US Preventive Services Task Force Recommendation on Prostate Cancer Screening
Abstract
Importance: In April 2017, the US Preventive Services Task Force (USPSTF) published a draft guideline that reversed its 2012 guidance advising against prostate-specific antigen (PSA)-based screening for prostate cancer in all men (grade D), instead endorsing individual decision-making for men aged 55 to 69 years (grade C).
Objective: To evaluate changes in rates of PSA testing after revisions in the USPSTF guideline on prostate cancer screening.
Design, setting, and participants: This retrospective cohort study used deidentified claims data from Blue Cross Blue Shield beneficiaries aged 40 to 89 years from January 1, 2013, through December 31, 2019.
Exposures: Publication of the USPSTF's draft (April 2017) and final (May 2018) recommendation on prostate cancer screening.
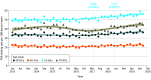
Main outcomes and measures: Age-adjusted rates of PSA testing in bimonthly periods were calculated, and PSA testing rates from calendar years before (January 1 to December 31, 2016) and after (January 1 to December 31, 2019) the guideline change were compared. Interrupted time series analyses were used to evaluate the association of the draft (April 2017) and published (May 2018) USPSTF guideline with rates of PSA testing. Changes in rates of PSA testing were further evaluated among beneficiaries within the age categories reflected in the guideline: 40 to 54 years, 55 to 69 years, and 70 to 89 years.
Results: The median number of eligible beneficiaries for each bimonthly period was 8 087 565 (range, 6 407 602-8 747 308), and the median age of all included eligible beneficiaries was 53 years (IQR, 47-59 years). Between 2016 and 2019, the mean (SD) rate of PSA testing increased from 32.5 (1.1) to 36.5 (1.1) tests per 100 person-years, a relative increase of 12.5% (95% CI, 1.1%-24.4%). During the same period, mean (SD) rates of PSA testing increased from 20.6 (0.8) to 22.7 (0.9) tests per 100 person-years among men aged 40 to 54 years (relative increase, 10.1%; 95% CI, -2.8% to 23.7%), from 49.8 (1.9) to 55.8 (1.8) tests per 100 person-years among men aged 55 to 69 years (relative increase, 12.1%; 95% CI, -0.2% to 25.2%), and from 38.0 (1.4) to 44.2 (1.4) tests per 100 person-years among men aged 70 to 89 years (relative increase, 16.2%; 95% CI, 4.2%-29.0%). Interrupted time series analysis revealed a significantly increasing trend of PSA testing after April 2017 among all beneficiaries (0.30 tests per 100 person-years for each bimonthly period; P < .001).
Conclusions and relevance: This large national cohort study found that rates of PSA testing increased after the USPSTF's draft statement in 2017, reversing trends seen after earlier guidance against PSA testing for all patients. Increased testing was also observed among older men, who may be less likely to benefit from prostate cancer screening.
Conflict of interest statement
Figures
Comment in
-
Prostate-Specific Antigen Testing for Prostate Cancer Screening-Is the Message Getting Through?JAMA Oncol. 2022 Jan 1;8(1):47-49. doi: 10.1001/jamaoncol.2021.5129. JAMA Oncol. 2022. PMID: 34762099 No abstract available.
-
Urological Oncology: Prostate Cancer.J Urol. 2022 Apr;207(4):928-930. doi: 10.1097/JU.0000000000002413. Epub 2022 Jan 7. J Urol. 2022. PMID: 34991328 No abstract available.
-
Re: Changes in Prostate-specific Antigen Testing Relative to the Revised US Preventive Services Task Force Recommendation on Prostate Cancer Screening.Eur Urol. 2022 Mar;81(3):313. doi: 10.1016/j.eururo.2021.12.028. Epub 2022 Jan 10. Eur Urol. 2022. PMID: 35027257 No abstract available.
References
-
- Chou R, Croswell JM, Dana T, et al. . Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(11):762-771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous