Questionnaires vs Interviews for the Assessment of Global Functional Outcomes After Traumatic Brain Injury
- PMID: 34762111
- PMCID: PMC8586906
- DOI: 10.1001/jamanetworkopen.2021.34121
Questionnaires vs Interviews for the Assessment of Global Functional Outcomes After Traumatic Brain Injury
Abstract
Importance: An interview is considered the gold standard method of assessing global functional outcomes in clinical trials among patients with acute traumatic brain injury (TBI). However, several multicenter clinical trials have used questionnaires completed by a patient or caregiver to assess the primary end point.
Objective: To examine agreement between interview and questionnaire formats for assessing TBI outcomes and to consider whether an interview has advantages.
Design, setting, and participants: This cohort study used data from patients enrolled in the Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI) project from December 2014 to December 2017. Data were analyzed from December 2020 to April 2021. Included patients were aged 16 years or older with TBI and a clinical indication for computed tomography imaging. Outcome assessments were completed using both an interview and a questionnaire at follow-up 3 and 6 months after injury.
Exposures: Traumatic brain injury of all severities.
Main outcomes and measures: Ratings on the Glasgow Outcome Scale-Extended (GOSE) administered as a structured interview rated by an investigator and as a questionnaire completed by patients or caregivers and scored centrally were compared, and the strength of agreement was evaluated using weighted κ statistics. Secondary outcomes included comparison of different sections of the GOSE assessments and the association of GOSE ratings with baseline factors and patient-reported mental health, health-related quality of life, and TBI symptoms.
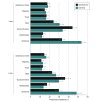
Results: Among the 3691 eligible individuals in the CENTER-TBI study, both GOSE assessment formats (interview and questionnaire) were completed by 994 individuals (26.9%) at 3 months after TBI (654 [65.8%] male; median age, 53 years [IQR, 33-66 years]) and 628 (17.0%) at 6 months (409 [65.1%] male; median age, 51 years [IQR, 31-64 years]). Outcomes of the 2 assessment methods agreed well at both 3 months (weighted κ, 0.77; 95% CI, 0.73-0.80) and 6 months (weighted κ, 0.82; 95% CI, 0.78-0.86). Furthermore, item-level agreement between the 2 methods was good for sections regarding independence in everyday activities (κ, 0.70-0.79 across both time points) and moderate for sections regarding subjective aspects of functioning such as relationships and symptoms (κ, 0.41-0.51 across both time points). Compared with questionnaires, interviews recorded more problems with work (294 [30.5%] vs 233 [24.2%] at 3 months and 161 [26.8%] vs 136 [22.7%] at 6 months), fewer limitations in social and leisure activities (330 [33.8%] vs 431 [44.1%] at 3 months and 179 [29.7%] vs 219 [36.4%] at 6 months), and more symptoms (524 [53.6%] vs 324 [33.1%] at 3 months and 291 [48.4%] vs 179 [29.8%] at 6 months). Interviewers sometimes assigned an overall rating based on judgment rather than interview scoring rules, particularly for patients with potentially unfavorable TBI outcomes. However, for both formats, correlations with baseline factors (ρ, -0.13 to 0.42) and patient-reported outcomes (ρ, 0.29 to 0.65) were similar in strength.
Conclusions and relevance: In this cohort study, GOSE ratings obtained by questionnaire and interview methods were in good agreement. The similarity of associations of the ratings obtained by both GOSE methods with baseline factors and other TBI outcome measures suggests that despite some apparent differences, the core information collected by both interviews and questionnaires was similar. The findings support the use of questionnaires in studies in which this form of contact may offer substantial practical advantages compared with interviews.
Conflict of interest statement
Figures
References
-
- Mendelow AD, Gregson BA, Rowan EN, et al. ; STITCH(Trauma) Investigators . Early surgery versus initial conservative treatment in patients with traumatic intracerebral hemorrhage (STITCH[Trauma]): the first randomized trial. J Neurotrauma. 2015;32(17):1312-1323. doi:10.1089/neu.2014.3644 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous