Denosumab in the Treatment of Osteoporosis: 10 Years Later: A Narrative Review
- PMID: 34762286
- PMCID: PMC8799550
- DOI: 10.1007/s12325-021-01936-y
Denosumab in the Treatment of Osteoporosis: 10 Years Later: A Narrative Review
Abstract
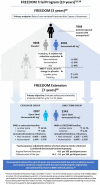
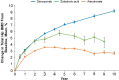
The fully human monoclonal antibody denosumab was approved for treatment of osteoporosis in 2010 on the basis of its potent antiresorptive activity, which produces clinically meaningful increases in bone mineral density (BMD) and reduces fracture risk at key skeletal sites. At that time, questions remained regarding the long-term safety and efficacy of this receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor; and with clinical experience, new questions have arisen regarding its optimal use. Here, we examine these questions through the lens of data from the FREEDOM trial program and other studies to determine where denosumab fits in the osteoporosis treatment landscape. Clinical consensus and evidentiary support have grown for denosumab as a highly effective anti-osteoporosis therapy for patients at high risk of fracture. In the 10-year FREEDOM Extension study, denosumab treatment produced progressive incremental increases in BMD, sustained low rates of vertebral fracture, and further reduction in nonvertebral fracture risk without increased risk of infection, cancer, or immunogenicity. There was no evidence that suppression of bone turnover or mineralization was excessive, and rates of osteonecrosis of the jaw (ONJ) and atypical femoral fracture (AFF) were very low. It is now recognized, however, that transitioning to another anti-osteoporosis therapy after denosumab discontinuation is essential to mitigate a transient rebound of bone turnover causing rapid BMD loss and increased risk of multiple vertebral fractures (MVFs). Taken together, the available data show that denosumab has a favorable benefit/risk profile and is a versatile agent for preventing osteoporotic fractures in the short and long term. Video abstract: Denosumab in the Treatment of Osteoporosis-10 Years Later (MP4 62727 KB).
Keywords: Bone density; Endocrinology; General medicine; Orthopedics; Osteoporosis; Therapeutics.
© 2021. The Author(s).
Figures


References
-
- Cosman F. Long-term treatment strategies for postmenopausal osteoporosis. Curr Opin Rheumatol. 2018;30:420–426. - PubMed
-
- Khosla S, Cauley JA, Compston J, et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res. 2017;32:424–430. - PubMed
-
- Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. - PubMed
-
- Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5:618–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

