The use of neutralizing monoclonal antibodies and risk of hospital admission and mortality in patients with COVID-19: a systematic review and meta-analysis of randomized trials
- PMID: 34762561
- PMCID: PMC8607536
- DOI: 10.1080/08923973.2021.1993894
The use of neutralizing monoclonal antibodies and risk of hospital admission and mortality in patients with COVID-19: a systematic review and meta-analysis of randomized trials
Abstract
Aim: Several randomized trials have evaluated the effect of neutralizing monoclonal antibodies on the risk of hospital admission and risk of mortality in patients with COVID-19. We aimed to summarize the overall evidence in the form of a systematic review and meta-analysis.
Methods: A systematic literature search with no language restriction was performed in electronic databases and preprint repositories to identify eligible studies published up to 29 June 2021. The outcomes of interest were hospital admission and all-cause mortality. A random-effects model was used to estimate the pooled odds ratio (OR) for outcomes of interest with the use of neutralizing monoclonal antibodies relative to nonuse of neutralizing monoclonal antibodies, at 95% confidence intervals (CI).
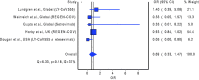
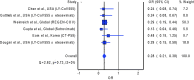
Results: Our systematic literature search identified nine randomized controlled trials. Three trials had an overall low risk of bias, while four trials had some concerns in the overall risk of bias. The meta-analysis revealed no statistically significant difference in the odds of mortality (pooled OR = 0.69; 95% CI 0.33-1.47), but a statistically significant reduction in the odds of hospital admission (pooled OR = 0.29; 95% CI 0.21-0.42), with the administration of a neutralizing monoclonal antibody among patients with COVID-19, relative to non-administration of a neutralizing monoclonal antibody, at the current sample size.
Conclusion: The reduced risk of hospital admission with neutralizing monoclonal antibodies use suggests that the timing of neutralizing antibodies administration is key in preventing hospital admission and, ultimately, death. Future randomized trials should aim to determine if the clinical outcomes with neutralizing monoclonal antibodies differ based on serostatus.
Keywords: Antibody; SARS-CoV-2; death; monoclonal; spike protein.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. - PubMed
-
- Voysey M, Clemens SAC, Madhi SA, et al. . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK [published correction appears in Lancet. 2021 Jan 9;397(10269):98]. Lancet. 2021;397(10269):99–111. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
