15LO1 dictates glutathione redox changes in asthmatic airway epithelium to worsen type 2 inflammation
- PMID: 34762602
- PMCID: PMC8718153
- DOI: 10.1172/JCI151685
15LO1 dictates glutathione redox changes in asthmatic airway epithelium to worsen type 2 inflammation
Abstract
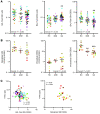
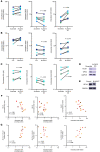
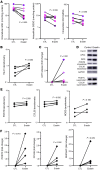
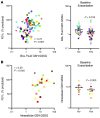
Altered redox biology challenges all cells, with compensatory responses often determining a cell's fate. When 15 lipoxygenase 1 (15LO1), a lipid-peroxidizing enzyme abundant in asthmatic human airway epithelial cells (HAECs), binds phosphatidylethanolamine-binding protein 1 (PEBP1), hydroperoxy-phospholipids, which drive ferroptotic cell death, are generated. Peroxidases, including glutathione peroxidase 4 (GPX4), metabolize hydroperoxy-phospholipids to hydroxy derivatives to prevent ferroptotic death, but consume reduced glutathione (GSH). The cystine transporter SLC7A11 critically restores/maintains intracellular GSH. We hypothesized that high 15LO1, PEBP1, and GPX4 activity drives abnormal asthmatic redox biology, evidenced by lower bronchoalveolar lavage (BAL) fluid and intraepithelial cell GSH:oxidized GSH (GSSG) ratios, to enhance type 2 (T2) inflammatory responses. GSH, GSSG (enzymatic assays), 15LO1, GPX4, SLC7A11, and T2 biomarkers (Western blot and RNA-Seq) were measured in asthmatic and healthy control (HC) cells and fluids, with siRNA knockdown as appropriate. GSSG was higher and GSH:GSSG lower in asthmatic compared with HC BAL fluid, while intracellular GSH was lower in asthma. In vitro, a T2 cytokine (IL-13) induced 15LO1 generation of hydroperoxy-phospholipids, which lowered intracellular GSH and increased extracellular GSSG. Lowering GSH further by inhibiting SLC7A11 enhanced T2 inflammatory protein expression and ferroptosis. Ex vivo, redox imbalances corresponded to 15LO1 and SLC7A11 expression, T2 biomarkers, and worsened clinical outcomes. Thus, 15LO1 pathway-induced redox biology perturbations worsen T2 inflammation and asthma control, supporting 15LO1 as a therapeutic target.
Keywords: Asthma; Metabolism; Pulmonology; Radicals; Th2 response.
Conflict of interest statement
Figures

Comment in
-
The role of 15 lipoxygenase 1 in asthma comes into focus.J Clin Invest. 2022 Jan 4;132(1):e155884. doi: 10.1172/JCI155884. J Clin Invest. 2022. PMID: 34981786 Free PMC article.
References
-
- Wang W, Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50(3):335–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

