Efficacy and safety of Bufei Huoxue capsules in the management of convalescent patients with COVID-19 infection: A multicentre, double-blind, and randomised controlled trial
- PMID: 34763045
- PMCID: PMC8575540
- DOI: 10.1016/j.jep.2021.114830
Efficacy and safety of Bufei Huoxue capsules in the management of convalescent patients with COVID-19 infection: A multicentre, double-blind, and randomised controlled trial
Abstract
Background: As of September 17, 2021, coronavirus disease 2019 (COVID-19) has infected more than 226 million people in a worldwide pandemic, with conservative estimates suggesting that there are more than 204 million convalescent patients with COVID-19. Previous studies have indicated that patients in the recovery phase exhibit decreased function of multiple organs. In China, traditional Chinese medicine (TCM) treatment is recommended in the rehabilitation period of COVID-19; however, the safety and efficacy of such treatment remain to be confirmed.
Aim of study: The present study aimed to evaluate the efficacy and safety of Bufei Huoxue (BFHX) in restoring the functional status and exercise tolerance of patients recovering from COVID-19.
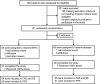
Methods: A total of 131 patients in the rehabilitation period of COVID-19 infection were randomly divided into a Bufei Huoxue (BFHX) group (n = 66) and a placebo group (n = 65). BFHX or placebo was given orally three times a day (1.4 g/dose) for 90 days. The primary outcomes was to evaluate improvements in exercise tolerance and imaging manifestations on chest computed tomography (CT).
Results: After the exclusion of two patients who withdrew prior to receiving any medications, 129 patients were recruited, including 64 patients in the BFHX group and 65 patients in the placebo group. After 3 months of treatment, the BFHX group exhibited greater attenuation of pneumonia lesions on chest CT than the placebo group (P<0.05). Improvements in 6-min walk distance (6MWD) relative to baseline were also significantly better in the BFHX group than in the placebo group (P<0.01). Scores on the Fatigue Assessment Inventory (FAI) were lower in the BFHX group than in the placebo group (P<0.05). Although the rate of adverse events was higher in the BFHX group than in the placebo group (9.38% vs. 4.62%), the difference was not significant (P=0.3241).
Conclusions: BFHX may exert strong rehabilitative effects on physiological activity in patients recovering from COVID-19, which may in turn attenuate symptoms of fatigue and improve exercise tolerance.
Keywords: Bufei Huoxue capsules; COVID-19 convalescence; Chinese Materia Medica; Randomised controlled trial.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Bai S.R., Wu Y., Wang Y., et al. Effect of bailing capsules combined with Bufei Huoxue capsules on pulmonary rehabilitation in patients with chronic obstructive pulmonary disease at stable stage of lung-kidney-qi deficiency syndrome. Chin. J. Exp. Trad. Med. Form. 2016;22:182–186. (in Chinese with English abstract)
-
- Beijing Administration of Traditional Chinese Medicine . 2020. Suggestions on TCM rehabilitation guidance of New Coronavirus Pneumonia in Convalescent Stage in Beijing (Trial)http://zyj.beijing.gov.cn/sy/tzgg/202003/t20200314_1706179.html
-
- Brooks D., Solway S., Gibbons W.J. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003;167:1287. - PubMed
-
- COVID-19 coronavirus pandemic 2021. https://www.worldometers.info/coronavirus/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

