COVID-19 vaccine - Long term immune decline and breakthrough infections
- PMID: 34763949
- PMCID: PMC8556595
- DOI: 10.1016/j.vaccine.2021.10.038
COVID-19 vaccine - Long term immune decline and breakthrough infections
Abstract
Background: Since the introduction of BNT162b2 mRNA COVID-19 vaccine by Pfizer in late 2020, efficacy and immunogenicity waning of COVID-19 vaccines was reported, and decision making regarding a booster remains a top priority worldwide, a decision that should be made based on breakthrough infection rate and antibody titer decline overtime.
Methods: We conducted a 5-month longitudinal prospective study involving vaccinated healthcare personnel, who were tested monthly for antibody titer, and sampled biweekly and on clinical indication for SARS-COV-2 polymerase chain reaction (PCR), to determine antibody decline and breakthrough infection.
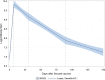
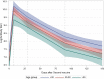
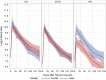
Results: 100 participants were recruited to the study. Antibody titer reached the climate after one month of the second dose of the vaccine, and declined rapidly thereafter: the median antibody levels were 895; 22,266; 9,682; 2,554 and 1,401 AU/ml in the day of the second dose, and in one month interval thereafter, respectively. In other words, four months after vaccination, the mean antibody level was 6% of the peak levels. During the study period, 4 breakthrough infections were diagnosed, 2 of which were asymptomatic, and the remaining two were mild cases; sharp elevation of antibody titer was seen after infection.
Conclusion: Antibody titer drops rapidly one month after the second dose of the vaccine. All infections within the study period were mild or asymptomatic, after which titer elevations were seen.
Keywords: Antibody; Booster; COVID; Immunogenicity; Vaccine.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

