Atezolizumab-induced scleroderma: a rare complication
- PMID: 34764112
- PMCID: PMC8586885
- DOI: 10.1136/bcr-2021-244968
Atezolizumab-induced scleroderma: a rare complication
Abstract
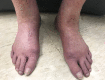
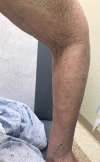
Few cases of programmed death-ligand 1 inhibitor-induced scleroderma have been reported and their clinical features remain unpublished. Optimal management is, therefore, unknown and an autoantibody association has yet to be identified. We present the case of a female in her 60s who developed skin thickening after starting atezolizumab for metastatic non-small cell lung cancer. Skin biopsy 7 months after symptom onset showed histological changes consistent with scleroderma. Anti-PM/SCL-75 antibody was positive. Atezolizumab was discontinued and treatment was started with mycophenolate mofetil. After 5 months, she experienced mild improvement in skin thickening. Earlier identification of this complication may limit morbidity in this disease process, which otherwise has limited treatment options. In suspected cases, obtaining scleroderma-associated autoantibodies may help with earlier diagnosis.
Keywords: chemotherapy; connective tissue disease; lung cancer (oncology); oncology; rheumatology.
© BMJ Publishing Group Limited 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. 10.1158/2159-8290.CD-18-0367 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous