Generation of functional ciliated cholangiocytes from human pluripotent stem cells
- PMID: 34764255
- PMCID: PMC8586142
- DOI: 10.1038/s41467-021-26764-0
Generation of functional ciliated cholangiocytes from human pluripotent stem cells
Abstract
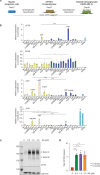
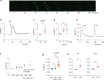
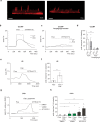
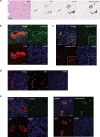
The derivation of mature functional cholangiocytes from human pluripotent stem cells (hPSCs) provides a model for studying the pathogenesis of cholangiopathies and for developing therapies to treat them. Current differentiation protocols are not efficient and give rise to cholangiocytes that are not fully mature, limiting their therapeutic applications. Here, we generate functional hPSC-derived cholangiocytes that display many characteristics of mature bile duct cells including high levels of cystic fibrosis transmembrane conductance regulator (CFTR) and the presence of primary cilia capable of sensing flow. With this level of maturation, these cholangiocytes are amenable for testing the efficacy of cystic fibrosis drugs and for studying the role of cilia in cholangiocyte development and function. Transplantation studies show that the mature cholangiocytes generate ductal structures in the liver of immunocompromised mice indicating that it may be possible to develop cell-based therapies to restore bile duct function in patients with biliary disease.
© 2021. The Author(s).
Conflict of interest statement
OHSU has commercially licensed HPd3/DHIC5-4D9; authors C.D. and M.G. are inventors of this antibody. The remaining authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

