Chronic pathophysiological changes in the normal brain parenchyma caused by radiotherapy accelerate glioma progression
- PMID: 34764346
- PMCID: PMC8585920
- DOI: 10.1038/s41598-021-01475-0
Chronic pathophysiological changes in the normal brain parenchyma caused by radiotherapy accelerate glioma progression
Abstract
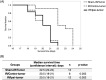
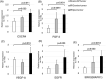
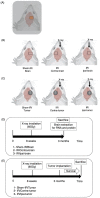
Radiation therapy is one of standard treatment for malignant glioma after surgery. The microenvironment after irradiation is considered not to be suitable for the survival of tumor cells (tumor bed effect). This study investigated whether the effect of changes in the microenvironment of parenchymal brain tissue caused by radiotherapy affect the recurrence and progression of glioma. 65-Gy irradiation had been applied to the right hemisphere of Fisher rats. After 3 months from irradiation, we extracted RNA and protein from the irradiated rat brain. To study effects of proteins extracted from the brains, we performed WST-8 assay and tube formation assay in vitro. Cytokine production were investigated for qPCR. Additionally, we transplanted glioma cell into the irradiated and sham animals and the median survival time of F98 transplanted rats was also examined in vivo. Immunohistochemical analyses and invasiveness of implanted tumor were evaluated. X-ray irradiation promoted the secretion of cytokines such as CXCL12, VEGF-A, TGF-β1 and TNFα from the irradiated brain. Proteins extracted from the irradiated brain promoted the proliferation and angiogenic activity of F98 glioma cells. Glioma cells implanted in the irradiated brains showed significantly high proliferation, angiogenesis and invasive ability, and the post-irradiation F98 tumor-implanted rats showed a shorter median survival time compared to the Sham-irradiation group. The current study suggests that the microenvironment around the brain tissue in the chronic phase after exposure to X-ray radiation becomes suitable for glioma cell growth and invasion.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370:709–722. - PubMed
-
- Anderson AP. Postoperative irradiation of glioblastomas. Results in a randomized series. Acta. Radiol. Oncol. Radiat. Phys. Biol. 1978;17(6):475–484. - PubMed
-
- Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N. Engl. J. Med. 1980;303(23):1323–1329. - PubMed
-
- Gasper LE, Fisher BJ, Macdonald DR, et al. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int. J. Radiat. Onco. Biol. Phys. 1992;24(1):55–57. - PubMed
-
- McDonald MW, Shu HK, Curran WJ, Jr, et al. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(1):130–136. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

