Nanoengineering of eco-friendly silver nanoparticles using five different plant extracts and development of cost-effective phenol nanosensor
- PMID: 34764386
- PMCID: PMC8586347
- DOI: 10.1038/s41598-021-01609-4
Nanoengineering of eco-friendly silver nanoparticles using five different plant extracts and development of cost-effective phenol nanosensor
Abstract
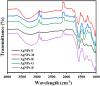
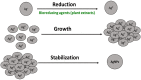
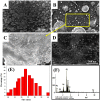
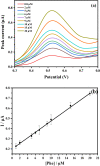
The production of environmentally friendly silver nanoparticles (AgNPs) has aroused the interest of the scientific community due to their wide applications mainly in the field of environmental pollution detection and water quality monitoring. Here, for the first time, five plant leaf extracts were used for the synthesis of AgNPs such as Basil, Geranium, Eucalyptus, Melia, and Ruta by a simple and eco-friendly method. Stable AgNPs were obtained by adding a silver nitrate (AgNO3) solution with the leaves extract as reducers, stabilizers and cappers. Only, within ten minutes of reaction, the yellow mixture changed to brown due to the reduction of Ag+ ions to Ag atoms. The optical, structural, and morphology characteristics of synthesized AgNPs were determined using a full technique like UV-visible spectroscopy, FTIR spectrum, XRD, EDX spectroscopy, and the SEM. Thus, Melia azedarach was found to exhibit smaller nanoparticles (AgNPs-M), which would be interesting for electrochemical application. So, a highly sensitive electrochemical sensor based on AgNPs-M modified GCE for phenol determination in water samples was developed, indicating that the AgNPs-M displayed good electrocatalytic activity. The developed sensor showed good sensing performances: a high sensitivity, a low LOD of 0.42 µM and good stability with a lifetime of about one month, as well as a good selectivity towards BPA and CC (with a deviation less than 10%) especially for nanoplastics analysis in the water contained in plastics bottles. The obtained results are repeatable and reproducible with RSDs of 5.49% and 3.18% respectively. Besides, our developed sensor was successfully applied for the determination of phenol in tap and mineral water samples. The proposed new approach is highly recommended to develop a simple, cost effective, ecofriendly, and highly sensitive sensor for the electrochemical detection of phenol which can further broaden the applications of green silver NPs.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Bao, Y. et al. Plant-extract-mediated synthesis of metal nanoparticles. J. Chem. 1–4, 6562687 (2021).
-
- Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008;10:507–517.
-
- Parashar, U. K., Saxena, P. S. & Srivastava, A. Bioinspired synthesis of silver nanoparticles. Digest J. Nanomater. Biostruct. (DJNB)4, 159–166 (2009).
-
- Tiwari, D. K., Behari, J. & Sen, P. Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Curr. Sci.95, 647–655 (2008).
-
- Wang Y, Xia Y. Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett. 2004;4:2047–2050.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

