Efficacy and Safety of Interferon-Alpha 2b for Patients with Hepatic Epithelioid Hemangioendothelioma: Outcomes of a Case-Series Analysis
- PMID: 34764690
- PMCID: PMC8572744
- DOI: 10.2147/CMAR.S334171
Efficacy and Safety of Interferon-Alpha 2b for Patients with Hepatic Epithelioid Hemangioendothelioma: Outcomes of a Case-Series Analysis
Abstract
Background: Hepatic epithelioid hemangioendothelioma (HEH) is a rare tumor type. No effective medicine or standard treatment for HEH has been established.
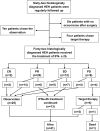
Patients and methods: From March 2014 to April 2021, 62 patients with pathologically diagnosed HEH were observed regularly, and interferon-alpha 2b (IFN-a 2b) was administered to patients with progressive disease or reoccurrence. Adverse events (AEs) were assessed and recorded, and a tumor assessment scan was performed every 3 months.
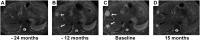
Results: A total of 42 patients with HEH received IFN-a 2b treatment in this study. No severe (grade ≥3) AEs were reported in the group overall. The most common treatment-related AEs in patients receiving IFN-a 2b were fever (50.0%) and fatigue (21.4%). Partial response and complete response were achieved in 20 patients (47.6%) and 2 patients (4.8%), respectively, and the objective response rate was 52.4%. Stable disease was observed in 12 patients (28.6%), and the disease control rate was 81.0%. Progressive disease was observed in 8 patients (19.0%). The 1-, 3-, and 5-year progression-free survival rates were 81.0%, 69.2%, and 62.3%, respectively. Only 1 patient died as a result of disease progression during the study. The 1-, 3-, and 5-year overall survival rates were 100%, 97.2%, and 97.2%, respectively.
Conclusion: IFN-a 2b is a safe and effective treatment for patients with HEH. The encouraging results with IFN-a 2b use make it a promising option for patients who have other types of epithelioid hemangioendothelioma; additional clinical trials are needed.
Keywords: chemotherapy; epithelioid hemangioendothelioma; interferon; liver; sarcoma.
© 2021 Liu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

