Discovery of a Series of Theophylline Derivatives Containing 1,2,3-Triazole for Treatment of Non-Small Cell Lung Cancer
- PMID: 34764872
- PMCID: PMC8576520
- DOI: 10.3389/fphar.2021.753676
Discovery of a Series of Theophylline Derivatives Containing 1,2,3-Triazole for Treatment of Non-Small Cell Lung Cancer
Abstract
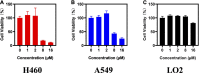
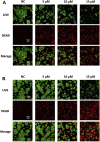
Chemotherapy is the most common clinical treatment for non-small cell lung cancer (NSCLC), but low efficiency and high toxicity of current chemotherapy drugs limit their clinical application. Therefore, it is urgent to develop hypotoxic and efficient chemotherapy drugs. Theophylline, a natural compound, is safe and easy to get, and it can be used as a modified scaffold structure and hold huge potential for developing safe and efficient antitumor drugs. Herein, we linked theophylline with different azide compounds to synthesize a new type of 1,2,3-triazole ring-containing theophylline derivatives. We found that some theophylline1,2,3-triazole compounds showed a good tumor-suppressive efficacy. Especially, derivative d17 showed strong antiproliferative activity against a variety of cancer cells in vitro, including H460, A549, A2780, LOVO, MB-231, MCF-7, OVCAR3, SW480, and PC-9. It is worth noting that the two NSCLC cell lines H460 H and A549 are sensitive to compound d17 particularly, with IC50 of 5.929 ± 0.97 μM and 6.76 ± 0.25 μM, respectively. Compound d17 can significantly induce cell apoptosis by increasing the ratio of apoptotic protein Bax/Bcl-2 by downregulating the expression of phosphorylated Akt protein, and it has little toxicity to normal hepatocyte cells LO2 at therapeutic concentrations. These data indicate that these theophylline acetic acid-1,2,3-triazole derivatives may be potential drug candidates for anti-NSCLC and are worthy of further study.
Keywords: 1,2,3-triazole; NSCLC; antitumor; apoptosis; theophylline.
Copyright © 2021 Ye, Mao, Xie, Zhang, Liu, Peng, Yang, Li and Yuan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Abou-Zied H. A., Youssif B. G. M., Mohamed M. F. A., Hayallah A. M., Abdel-Aziz M. (2019). EGFR inhibitors and apoptotic inducers: Design, synthesis, anticancer activity and docking studies of novel xanthine derivatives carrying chalcone moiety as hybrid molecules. Bioorg. Chem. 89, 102997. 10.1016/j.bioorg.2019.102997 - DOI - PubMed
-
- Al-Blewi F. F., Almehmadi M. A., Aouad M. R., Bardaweel S. K., Sahu P. K., Messali M., et al. (2018). Design, synthesis, ADME prediction and pharmacological evaluation of novel benzimidazole-1,2,3-triazole-sulfonamide hybrids as antimicrobial and antiproliferative agents. Chem. Cent. J. 12 (1), 110. 10.1186/s13065-018-0479-1 - DOI - PMC - PubMed
-
- Aouad M. R., Khan D. J. O., Said M. A., Al-Kaff N. S., Rezki N., Ali A. A., et al. (2021). Novel 1,2,3-Triazole Derivatives as Potential Inhibitors against Covid-19 Main Protease: Synthesis, Characterization, Molecular Docking and DFT Studies. Chemistry Select 6 (14), 3468–3486. 10.1002/slct.202100522 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

