Clostridoides difficile Infection Severity Assessment by Fecal Calprotectin: A Pilot Study
- PMID: 34765239
- PMCID: PMC8551903
- DOI: 10.12865/CHSJ.47.02.09
Clostridoides difficile Infection Severity Assessment by Fecal Calprotectin: A Pilot Study
Abstract
Clostridoides difficile infection (CDI) is the leading cause of antibiotic related diarrhea therapy and may associate high morbidity and mortality. Providing a potential biomarker to assess disease severity may help physicians in choosing the right treatment.
Methods: This was a prospective, single-centre cohort study which included patients diagnosed with CDI which were assessed by fecal calprotectin (FC).
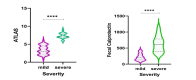
Results: Patients included had a mean of 69.29 years of age, 54.23% of male gender. Patients diagnosed with mild CDI had a mean ATLAS score of 3.39 (±1.24), statistically lower (p<0.001) than patients with severe CDI who had a mean ATLAS score of 7.33 (±0.77). Fecal calprotectin concentrations were significantly higher (p<0.001) in the severe CDI patients (615.14μg/g; IQR, 403.62-784.4μg/g) than in the mild CDI patients (195.42μg/g; IQR, 131.12-298.59μg/g). We suggest a cut-off of 290.09μg/g for the predictive marker of fecal calprotectin, which permitted to identify patients with severe and mild CDI, having 100% sensitivity and 76% specificity.
Conclusions: Our findings point out the potential that FC might have, as a biomarker for disease severity. However, future multicentre studies and in larger cohort need to validate the results.
Keywords: Clostridoides difficile infection; fecal calprotectin; predictive value.
Copyright © 2014, Medical University Publishing House Craiova.
Conflict of interest statement
None to declare.
Figures
References
-
- Martin JS, Monaghan TM, Wilcox MH. Clostridium difficile infection: epidemiology, diagnosis and understanding transmission. Nat Rev Gastroenterol Hepatol. 2016;13(4):206–216. - PubMed
-
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH, Society for Healthcare epidemiology of America Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. - PubMed
LinkOut - more resources
Full Text Sources