Synthesis, 3D-QSAR and Molecular Docking Study of Nopol-Based 1,2,4-Triazole-Thioether Compounds as Potential Antifungal Agents
- PMID: 34765587
- PMCID: PMC8576812
- DOI: 10.3389/fchem.2021.757584
Synthesis, 3D-QSAR and Molecular Docking Study of Nopol-Based 1,2,4-Triazole-Thioether Compounds as Potential Antifungal Agents
Abstract
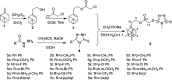
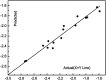
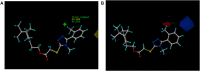
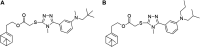
Cytochrome bc 1 complex is an important component of cellular respiratory chain, and it is also an important target enzyme to inhibit the growth of plant pathogens. Using cytochrome bc 1 complex as the target enzyme, twenty-three novel nopol-based 1,2,4-triazole-thioether compounds were designed and synthesized from natural preponderant resource β-pinene, and their structures were confirmed by FT-IR, NMR, ESI-MS and elemental analysis. The in vitro antifungal activity of the target compounds 5a-5w was preliminarily evaluated against eight plant pathogens at the concentration of 50 µg/ml. The bioassay results showed that the target compounds exhibited the best antifungal activity against Physalospora piricola, in which compounds 5b (R= o-CH3 Ph), 5e (R= o-OCH3 Ph), 5h (R= o-F Ph), 5m (R= o-Br Ph), 5o (R= m,m-OCH3 Ph), and 5r (R= p-OH Ph) had inhibition rates of 91.4, 83.3, 86.7, 83.8, 91.4 and 87.3%, respectively, much better than that of the positive control chlorothalonil. Also, compound 5a (R= Ph) had inhibition rate of 87.9% against Rhizoeotnia solani, and compound 5b (R= o-CH3 Ph) had inhibition rates of 87.6 and 89% against Bipolaris maydis and Colleterichum orbicala, respectively. In order to develop novel and promising antifungal compounds against P. piricola, the analysis of three-dimensional quantitative structure-activity relationship (3D-QSAR) was carried out using the CoMFA method on the basis of their antifungal activity data, and a reasonable and effective 3D-QSAR model (r 2 = 0.944, q 2 = 0.685) has been established. In addition, the theoretical study of molecular docking revealed that the target compounds could bind to and interact with the site of cytochrome bc 1 complex.
Keywords: 1, 2, 4-triazole-thioether; 3D-QSAR; molecular docking; nopol; β-pinene.
Copyright © 2021 Wang, Duan, Lin, Li, Chen and Lei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Synthesis, Antifungal Activity, 3D-QSAR, and Molecular Docking Study of Novel Menthol-Derived 1,2,4-Triazole-thioether Compounds.Molecules. 2021 Nov 17;26(22):6948. doi: 10.3390/molecules26226948. Molecules. 2021. PMID: 34834038 Free PMC article.
-
Synthesis, Antifungal Activity, and 3D-QSAR Study of Novel Nopol-Derived 1,3,4-Thiadiazole-Thiourea Compounds.Molecules. 2021 Mar 18;26(6):1708. doi: 10.3390/molecules26061708. Molecules. 2021. PMID: 33803890 Free PMC article.
-
Synthesis of Bioactive Compounds from 3-Carene (II): Synthesis, Antifungal Activity and 3D-QSAR Study of (Z)- and (E)-3-Caren-5-One Oxime Sulfonates.Molecules. 2019 Jan 29;24(3):477. doi: 10.3390/molecules24030477. Molecules. 2019. PMID: 30699975 Free PMC article.
-
Synthesis, antifungal activity and 3D-QSAR study of novel acyl thiourea compounds containing gem-dimethylcyclopropane ring.Mol Divers. 2022 Feb;26(1):125-136. doi: 10.1007/s11030-020-10163-6. Epub 2021 Apr 29. Mol Divers. 2022. PMID: 33914211
-
Synthesis and Antifungal Activity of Novel 3-Caren-5-One Oxime Esters.Molecules. 2017 Sep 12;22(9):1538. doi: 10.3390/molecules22091538. Molecules. 2017. PMID: 28895932 Free PMC article.
Cited by
-
Synthesis, Characterization, DFT Mechanistic Study, Antimicrobial Activity, Molecular Modeling, and ADMET Properties of Novel Pyrazole-isoxazoline Hybrids.ACS Omega. 2022 Dec 8;7(50):46731-46744. doi: 10.1021/acsomega.2c05788. eCollection 2022 Dec 20. ACS Omega. 2022. PMID: 36570248 Free PMC article.
-
Synthesis of Benzimidazole-1,2,4-triazole Derivatives as Potential Antifungal Agents Targeting 14α-Demethylase.ACS Omega. 2023 Jan 19;8(4):4369-4384. doi: 10.1021/acsomega.2c07755. eCollection 2023 Jan 31. ACS Omega. 2023. PMID: 36743066 Free PMC article.
-
2-Amino Thiazole Derivatives as Prospective Aurora Kinase Inhibitors against Breast Cancer: QSAR, ADMET Prediction, Molecular Docking, and Molecular Dynamic Simulation Studies.ACS Omega. 2023 Nov 7;8(46):44287-44311. doi: 10.1021/acsomega.3c07003. eCollection 2023 Nov 21. ACS Omega. 2023. PMID: 38027360 Free PMC article.
-
Synthesis and Antitumor Evaluation of Menthone-Derived Pyrimidine-Urea Compounds as Potential PI3K/Akt/mTOR Signaling Pathway Inhibitor.Front Chem. 2022 Feb 3;9:815531. doi: 10.3389/fchem.2021.815531. eCollection 2021. Front Chem. 2022. PMID: 35186896 Free PMC article.
-
A Deep Learning-Based Quantitative Structure-Activity Relationship System Construct Prediction Model of Agonist and Antagonist with High Performance.Int J Mol Sci. 2022 Feb 15;23(4):2141. doi: 10.3390/ijms23042141. Int J Mol Sci. 2022. PMID: 35216254 Free PMC article.
References
-
- Abdel-Aziz M., Beshr E. A., Abdel-Rahman I. M., Ozadali K., Tan O. U., Aly O. M. (2014). 1-(4-Methoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazole-3-carboxamides: Synthesis, Molecular Modeling, Evaluation of Their Anti-inflammatory Activity and Ulcerogenicity. Eur. J. Med. Chem. 77, 155–165. 10.1016/j.ejmech.2014.03.001 - DOI - PubMed
-
- Chen C., Wu Q. Y., Shan L. Y., Zhang B., Verpoort F., Yang G. F. (2016). Discovery of Cytochrome Bc 1 Complex Inhibitors Inspired by the Natural Product Karrikinolide. RSC Adv. 6, 97580–97586. 10.1039/c6ra19424a - DOI
-
- Chen J. Z., Xiao Z. Q., Xu L. F., Wang Z. D. (2017). The Synthesis and Antifungal of Hydronopyl Alkylamine and its Acetylation Derivative. Chem. Res. Appl. 29, 1728–1732. 10.14233/ajchem.2017.20224 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

