Late Gadolinium Enhancement Cardiovascular Magnetic Resonance Assessment of Substrate for Ventricular Tachycardia With Hemodynamic Compromise
- PMID: 34765656
- PMCID: PMC8576410
- DOI: 10.3389/fcvm.2021.744779
Late Gadolinium Enhancement Cardiovascular Magnetic Resonance Assessment of Substrate for Ventricular Tachycardia With Hemodynamic Compromise
Abstract
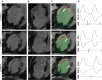
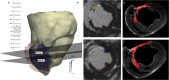
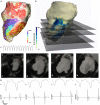
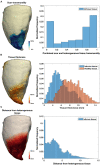
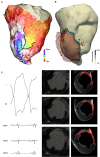
Background: The majority of data regarding tissue substrate for post myocardial infarction (MI) VT has been collected during hemodynamically tolerated VT, which may be distinct from the substrate responsible for VT with hemodynamic compromise (VT-HC). This study aimed to characterize tissue at diastolic locations of VT-HC in a porcine model. Methods: Late Gadolinium Enhancement (LGE) cardiovascular magnetic resonance (CMR) imaging was performed in eight pigs with healed antero-septal infarcts. Seven pigs underwent electrophysiology study with venous arterial-extra corporeal membrane oxygenation (VA-ECMO) support. Tissue thickness, scar and heterogeneous tissue (HT) transmurality were calculated at the location of the diastolic electrograms of mapped VT-HC. Results: Diastolic locations had median scar transmurality of 33.1% and a median HT transmurality 7.6%. Diastolic activation was found within areas of non-transmural scar in 80.1% of cases. Tissue activated during the diastolic component of VT circuits was thinner than healthy tissue (median thickness: 5.5 mm vs. 8.2 mm healthy tissue, p < 0.0001) and closer to HT (median distance diastolic tissue: 2.8 mm vs. 11.4 mm healthy tissue, p < 0.0001). Non-scarred regions with diastolic activation were closer to steep gradients in thickness than non-scarred locations with normal EGMs (diastolic locations distance = 1.19 mm vs. 9.67 mm for non-diastolic locations, p < 0.0001). Sites activated late in diastole were closest to steep gradients in tissue thickness. Conclusions: Non-transmural scar, mildly decreased tissue thickness, and steep gradients in tissue thickness represent the structural characteristics of the diastolic component of reentrant circuits in VT-HC in this porcine model and could form the basis for imaging criteria to define ablation targets in future trials.
Keywords: cardiovascular magnetic resonance; late gadolinium enhancement; mechanical circulatory support; venous-arterial extra corporeal membrane oxygenation (VA-ECMO); ventricular tachycardia.
Copyright © 2021 Whitaker, Neji, Kim, Connolly, Aubriot, Calvo, Karim, Roney, Murfin, Richardson, Morgan, Ismail, Harrison, de Vos, Aalders, Williams, Mukherjee, O'Neill, Chubb, Tschabrunn, Anter, Camporota, Niederer, Roujol, Bishop, Wright, Silberbauer, Razavi and O'Neill.
Conflict of interest statement
The CardioHelp machine and all ECMO consumables were donated by Maquet, Getinge Group without conditions on their use. The Precision and Claris system and all EP mapping consumables were donated by Abbott without conditions on their use. The authors declare a potential conflict of interest and state it below. RN was employed by the company Siemens Healthcare. SK and TA were employed by the company Abbott Medical. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

