Emergence of cationic polyamine dendrimersomes: design, stimuli sensitivity and potential biomedical applications
- PMID: 34765868
- PMCID: PMC8548884
- DOI: 10.1039/d1na00536g
Emergence of cationic polyamine dendrimersomes: design, stimuli sensitivity and potential biomedical applications
Abstract
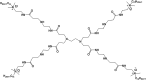
For decades, self-assembled lipid vesicles have been widely used in clinics as nanoscale delivery systems for various biomedical applications, including treatment of various diseases. Due to their core-shell architecture and versatile nature, they have been successfully used as carriers for the delivery of a wide range of therapeutic cargos, including drugs and nucleic acids, in cancer treatment. Recently, surface-modified polyamine dendrimer-based vesicles, or dendrimersomes, have emerged as promising alternatives to lipid vesicles for various biomedical applications, due to their ease of synthesis, non-immunogenicity, stability in circulation and lower size polydispersity. This mini-review provides an overview of the recent advances resulting from the use of biomimetic hydrophobically-modified polyamine-based dendrimersomes towards biomedical applications, focusing mainly on the two most widely used polyamine dendrimers, namely polyamidoamine (PAMAM) and poly(propylene imine) (PPI) dendrimers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


















References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

