Nanotechnology-enhanced immunotherapy for metastatic cancer
- PMID: 34766099
- PMCID: PMC8571799
- DOI: 10.1016/j.xinn.2021.100174
Nanotechnology-enhanced immunotherapy for metastatic cancer
Abstract
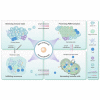
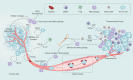
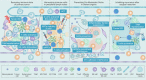
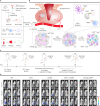
A vast majority of cancer deaths occur as a result of metastasis. Unfortunately, effective treatments for metastases are currently lacking due to the difficulty of selectively targeting these small, delocalized tumors distributed across a variety of organs. However, nanotechnology holds tremendous promise for improving immunotherapeutic outcomes in patients with metastatic cancer. In contrast to conventional cancer immunotherapies, rationally designed nanomaterials can trigger specific tumoricidal effects, thereby improving immune cell access to major sites of metastasis such as bone, lungs, and lymph nodes, optimizing antigen presentation, and inducing a persistent immune response. This paper reviews the cutting-edge trends in nano-immunoengineering for metastatic cancers with an emphasis on different nano-immunotherapeutic strategies. Specifically, it discusses directly reversing the immunological status of the primary tumor, harnessing the potential of peripheral immune cells, preventing the formation of a pre-metastatic niche, and inhibiting the tumor recurrence through postoperative immunotherapy. Finally, we describe the challenges facing the integration of nanoscale immunomodulators and provide a forward-looking perspective on the innovative nanotechnology-based tools that may ultimately prove effective at eradicating metastatic diseases.
Keywords: immunomodulators; immunotherapy; metastatic cancer; nanomaterials; tumor microenvironments.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- He B., Johansson-Percival A., Backhouse J., et al. Remodeling of metastatic vasculature reduces lung colonization and sensitizes overt metastases to immunotherapy. Cell Rep. 2020;30:714–724. e715. - PubMed
-
- Schroeder A., Heller D.A., Winslow M.M., et al. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer. 2012;12:39–50. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

