Lipid metabolism in cancer progression and therapeutic strategies
- PMID: 34766135
- PMCID: PMC8491217
- DOI: 10.1002/mco2.27
Lipid metabolism in cancer progression and therapeutic strategies
Abstract
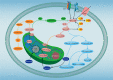
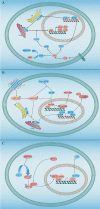
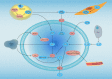
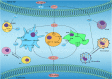
Dysregulated lipid metabolism represents an important metabolic alteration in cancer. Fatty acids, cholesterol, and phospholipid are the three most prevalent lipids that act as energy producers, signaling molecules, and source material for the biogenesis of cell membranes. The enhanced synthesis, storage, and uptake of lipids contribute to cancer progression. The rewiring of lipid metabolism in cancer has been linked to the activation of oncogenic signaling pathways and cross talk with the tumor microenvironment. The resulting activity favors the survival and proliferation of tumor cells in the harsh conditions within the tumor. Lipid metabolism also plays a vital role in tumor immunogenicity via effects on the function of the noncancer cells within the tumor microenvironment, especially immune-associated cells. Targeting altered lipid metabolism pathways has shown potential as a promising anticancer therapy. Here, we review recent evidence implicating the contribution of lipid metabolic reprogramming in cancer to cancer progression, and discuss the molecular mechanisms underlying lipid metabolism rewiring in cancer, and potential therapeutic strategies directed toward lipid metabolism in cancer. This review sheds new light to fully understanding of the role of lipid metabolic reprogramming in the context of cancer and provides valuable clues on therapeutic strategies targeting lipid metabolism in cancer.
Keywords: cancer; lipid metabolism; mechanism; microenvironment; therapeutic strategy.
© 2020 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309‐314. - PubMed
-
- Rohrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16(11):732‐749. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
