Clinical benefit of neoadjuvant anti-PD-1/PD-L1 utilization among different tumors
- PMID: 34766136
- PMCID: PMC8491227
- DOI: 10.1002/mco2.61
Clinical benefit of neoadjuvant anti-PD-1/PD-L1 utilization among different tumors
Abstract
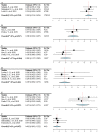
PD-1/PD-L1 (programmed cell death-1 and programmed death-ligand 1) inhibitors utilization in neoadjuvant therapy has been assessed in tumors. This study focused on the clinical benefits of neoadjuvant anti-PD-1/PD-L1 therapy. A comprehensive search was conducted in electronic databases to identify eligible studies. Major response rate (MRR) and complete response rate (CRR) were pooled in this analysis to assess the efficacy of neoadjuvant anti-PD-1/PD-L1 utilization, all grades and high-grade adverse events (AEs) were pooled to evaluate its safety. Twenty studies were included in this meta-analysis, with 828 patients suffering from different tumors. The pooled CRR of triple-negative breast cancer was 0.569 (95% CI 0.514, 0.624, I 2 = 0%) and the pooled MRR of lung cancer was 0.471 (95% CI 0.267, 0.575, I 2 = 0%). The most frequent adverse event was fatigue (0.272 95% CI 0.171, 0.402, I 2 = 87%), and the most common high-grade adverse event was febrile neutropenia (0.084 95% CI 0.063, 0.112, I 2 = 85%). In conclusion, neoadjuvant anti-PD-1/PD-L1 therapy received satisfactory clinical results in these tumors included.
Keywords: PD‐1/PD‐L1 inhibitors; atezolizumab; lung cancer; neoadjuavant therapy; triple‐negative breast cancer.
© 2021 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
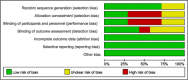
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Kaufmann M, Hortobagyi GN, Goldhirsch A, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24(12):1940–1949. - PubMed
-
- Bielack SS, Kempf‐Bielack B, Delling G, et al. Prognostic factors in high‐grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–790. - PubMed
-
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866. - PubMed
-
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
