Dilation of cortical capillaries is not related to astrocyte calcium signaling
- PMID: 34767261
- PMCID: PMC8732319
- DOI: 10.1002/glia.24119
Dilation of cortical capillaries is not related to astrocyte calcium signaling
Abstract
The brain requires an adequate supply of oxygen and nutrients to maintain proper function as neuronal activity varies. This is achieved, in part, through neurovascular coupling mechanisms that mediate local increases in blood flow through the dilation of arterioles and capillaries. The role of astrocytes in mediating this functional hyperemia response is controversial. Specifically, the function of astrocyte Ca2+ signaling is unclear. Cortical arterioles dilate in the absence of astrocyte Ca2+ signaling, but previous work suggests that Ca2+ increases are necessary for capillary dilation. This question has not been fully addressed in vivo, however, and we have reexamined the role of astrocyte Ca2+ signaling in vessel dilation in the barrel cortex of awake, behaving mice. We recorded evoked vessel dilations and astrocyte Ca2+ signaling in response to whisker stimulation. Experiments were carried out on WT and IP3R2 KO mice, a transgenic model where astrocyte Ca2+ signaling is substantially reduced. Compared to WT mice at rest, Ca2+ signaling in astrocyte endfeet contacting capillaries increased by 240% when whisker stimulation evoked running. In contrast, Ca2+ signaling was reduced to 9% of WT values in IP3R2 KO mice. In all three conditions, however, the amplitude of capillary dilation was largely unchanged. In addition, the latency to the onset of astrocyte Ca2+ signaling lagged behind dilation onset in most trials, although a subset of rapid onset Ca2+ events with latencies as short as 0.15 s occurred. In summary, we found that whisker stimulation-evoked capillary dilations occurred independent of astrocyte Ca2+ increases in the cerebral cortex.
Keywords: IP3R2 KO; astrocyte; awake mouse; calcium signaling; capillary dilation; cerebral blood flow; endfeet; processes.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST. The authors declare no conflict of interest.
Figures
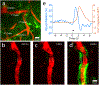

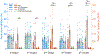
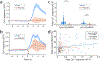

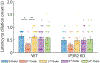
References
-
- Bazargani N, Attwell D. 2016. Astrocyte calcium signaling: the third wave. Nat Neurosci 19:182–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

