Blinatumomab Nonresponse and High-Disease Burden Are Associated With Inferior Outcomes After CD19-CAR for B-ALL
- PMID: 34767461
- PMCID: PMC8937010
- DOI: 10.1200/JCO.21.01405
Blinatumomab Nonresponse and High-Disease Burden Are Associated With Inferior Outcomes After CD19-CAR for B-ALL
Abstract
Purpose: CD19-targeted chimeric antigen receptor T cells (CD19-CAR) and blinatumomab effectively induce remission in relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) but are also associated with CD19 antigen modulation. There are limited data regarding the impact of prior blinatumomab exposure on subsequent CD19-CAR outcomes.
Patients and methods: We conducted a multicenter, retrospective review of children and young adults with relapsed or refractory ALL who received CD19-CAR between 2012 and 2019. Primary objectives addressed 6-month relapse-free survival (RFS) and event-free survival (EFS), stratified by blinatumomab use. Secondary objectives included comparison of longer-term survival outcomes, complete remission rates, CD19 modulation, and identification of factors associated with EFS.
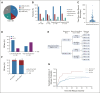
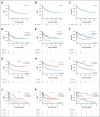
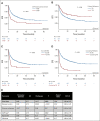
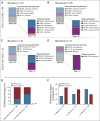
Results: Of 420 patients (median age, 12.7 years; interquartile range, 7.1-17.5) treated with commercial tisagenlecleucel or one of three investigational CD19-CAR constructs, 77 (18.3%) received prior blinatumomab. Blinatumomab-exposed patients more frequently harbored KMT2A rearrangements and underwent a prior stem-cell transplant than blinatumomab-naïve patients. Among patients evaluable for CD19-CAR response (n = 412), blinatumomab nonresponders had lower complete remission rates to CD19-CAR (20 of 31, 64.5%) than blinatumomab responders (39 of 42, 92.9%) or blinatumomab-naive patients (317 of 339, 93.5%), P < .0001. Following CD19-CAR, blinatumomab nonresponders had worse 6-month EFS (27.3%; 95% CI, 13.6 to 43.0) compared with blinatumomab responders (66.9%; 95% CI, 50.6 to 78.9; P < .0001) or blinatumomab-naïve patients (72.6%; 95% CI, 67.5 to 77; P < .0001) and worse RFS. High-disease burden independently associated with inferior EFS. CD19-dim or partial expression (preinfusion) was more frequently seen in blinatumomab-exposed patients (13.3% v 6.5%; P = .06) and associated with lower EFS and RFS.
Conclusion: With the largest series to date in pediatric CD19-CAR, and, to our knowledge, the first to study the impact of sequential CD19 targeting, we demonstrate that blinatumomab nonresponse and high-disease burden were independently associated with worse RFS and EFS, identifying important indicators of long-term outcomes following CD19-CAR.
Conflict of interest statement
Figures
Comment in
-
Taking a BiTE Out of CAR-T Cell Efficacy.J Clin Oncol. 2022 Mar 20;40(9):921-923. doi: 10.1200/JCO.21.02465. Epub 2021 Nov 12. J Clin Oncol. 2022. PMID: 34767437 Free PMC article. No abstract available.
References
-
- Grupp S, Maude S, Rives S, et al. : Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (r/r) acute lymphoblastic leukemia. Blood abstract 895, ASH Meeting, 2019
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

