Prostate-specific membrane antigen (PSMA) fusion imaging in prostate cancer: PET-CT vs PET-MRI
- PMID: 34767482
- PMCID: PMC8978229
- DOI: 10.1259/bjr.20210728
Prostate-specific membrane antigen (PSMA) fusion imaging in prostate cancer: PET-CT vs PET-MRI
Abstract
Objectives: To investigate whether PET-CT or PET-MRI is more appropriate for imaging prostate cancer, in terms of primary tumor detection, local staging and recurrence, as well as lymph nodes and distant metastases.
Methods: A systematic literature search was conducted on Embase, PubMed/MEDLINE, and the Cochrane Library database. Studies evaluating the diagnostic performance of PET-CT vs PET-MRI in prostate cancer patients were emphasized.
Results: We reviewed 57 original research articles during the period 2016-2021: 14 articles regarding the radiotracer PSMA; 18 articles regarding the primary tumor detection, local tumor staging, managing local recurrence; 17 articles for managing lymph node metastases; and eight articles for managing bone and other distant metastases. PSMA PET could be complementary to mpMRI for primary prostate cancer localization and is particularly valuable for PI-RADS three lesions. PET-MRI is better than PET-CT in local tumor staging due to its specific benefit in predicting extracapsular extension in MRI-occult prostate cancer patients. PET-MRI is likely superior as compared with PET-CT in detecting local recurrence, and has slightly higher detection rates than PET-CT in lymph node recurrence. PET-CT and PET-MRI seem to have equivalent performance in detecting distant bony or visceral metastases.
Conclusion: In conclusion, PET-MRI is suitable for local and regional disease, either primary staging or restaging, whereas PET-CT is valuable for managing distant bony or visceral metastasis.
Advances in knowledge: We reviewed the emerging applications of PET-MRI and PET-CT in clinical aspects. Readers will gain an objective overview on the strength and shortfalls of PET-MRI or PET-CT in the management of prostate cancer.
Figures

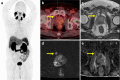
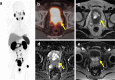
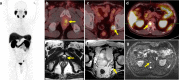
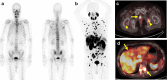
References
-
- Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol 2016; 195: 1436–43. doi: 10.1016/j.juro.2015.12.025 - DOI - PubMed
-
- Kesch C, Vinsensia M, Radtke JP, Schlemmer HP, Heller M, Ellert E, et al. Intraindividual cComparison of 18F-PSMA-1007 PET–CT, mMultiparametric MRI, and radical prostatectomy specimens in patients with primary prostate cancer: a retrospective, proof-of-concept studyRadical Prostatectomy Specimens in Patients with Primary Prostate Cancer: A Retrospective, Proof-of-Concept Study. J Nucl Med 2017; 58: 1805–10. doi: 10.2967/jnumed.116.189233 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

