Novel Pressure Wave Separation Analysis for Cardiovascular Function Assessment Highlights Major Role of Aortic Root
- PMID: 34767501
- PMCID: PMC7612937
- DOI: 10.1109/TBME.2021.3127799
Novel Pressure Wave Separation Analysis for Cardiovascular Function Assessment Highlights Major Role of Aortic Root
Abstract
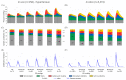
Objective: A novel method was presented to separate the central blood pressure wave (CBPW) into five components with different biophysical and temporal origins. It includes a time-varying emission coefficient ( γ) that quantifies pulse wave generation and reflection at the aortic root.
Methods: The method was applied to normotensive subjects with modulated physiology by inotropic/vasoactive drugs (n = 13), hypertensive subjects (n = 158), and virtual subjects (n = 4,374).
Results: γ is directly proportional to aortic flow throughout the cardiac cycle. Mean peak γ increased with increasing pulse pressure (from <30 to >70 mmHg) in the hypertensive (from 1.6 to 2.5, P < 0.001) and in silico (from 1.4 to 2.8, P < 0.001) groups, dobutamine dose (from baseline to 7.5 μg/kg/min) in the normotensive group (from 2.1 to 2.7, P < 0.05), and remained unchanged when peripheral wave reflections were suppressed in silico. This was accompanied by an increase in the percentage contribution of the cardiac-aortic-coupling component of CBPW in systole: from 11% to 23% (P < 0.001) in the hypertensive group, 9% to 21% (P < 0.001) in the in silico group, and 17% to 23% (P < 0.01) in the normotensive group.
Conclusion: These results suggest that the aortic root is a major reflection site in the systemic arterial network and ventricular-aortic coupling is the main determinant in the elevation of pulsatile pulse pressure.
Significance: Ventricular-aortic coupling is a prime therapeutic target for preventing/treating systolic hypertension.
Figures




References
-
- O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45(4):652–658. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

