Different virulence levels of Enterococcus cecorum strains in experimentally infected meat-type chickens
- PMID: 34767595
- PMCID: PMC8589174
- DOI: 10.1371/journal.pone.0259904
Different virulence levels of Enterococcus cecorum strains in experimentally infected meat-type chickens
Abstract
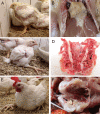
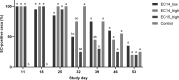
In recent years, pathogenic strains of Enterococcus cecorum (EC) have emerged as a causing agent of septicemia and skeletal infection in broiler chickens with a high economic impact worldwide. Although research has been conducted, many aspects of the pathogenesis of the EC-associated disease are still unknown. In the present study, an experimental infection model was established in broiler chickens. Two different EC strains (EC14 and EC15) were compared in two different concentrations of each strain (2 × 106 and 2 × 108 colony-forming units per milliliter (CFU/mL)) after oral infection of one-day-old chicks. Clinical signs and gross lesions of the EC-associated disease were monitored in the following seven weeks. Although both EC strains were originally isolated from clinical disease outbreaks and had a high embryonic lethality, only EC14 successfully induced the typical course of the EC-associated disease with characteristic clinical signs and gross lesions. In total, 23% of the birds in the two EC14-groups were EC-positive in extraintestinal organs on culture, and no differences were found between the two infectious doses. EC14 was frequently detected via real-time PCR in the free thoracic vertebra (FTV) and femoral heads without any detectable gross lesions. The number of EC positive spleens from infected broilers was comparable using bacterial isolation and a specific real-time PCR. Interestingly, EC15 was not detected in extraintestinal organs, although birds in the EC15 groups were colonized by EC in the ceca after experimental infection. The present study represents first proof that virulence differs among EC strains in experimentally infected chickens, and emphasizes the need to further characterize virulence factors and pathogenic mechanisms of EC. The strain EC14 at a dose of 106 CFU is suitable for reproduction of the EC-associated disease. The experimental infection model reported here provides the basis for further research on the EC pathogenesis and possible prevention and intervention strategies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Devriese LA, Dutta GN, Farrow JAE, Vandekerckhove A, Phillips BA. Streptococcus-Cecorum, a New Species Isolated from Chickens. Int J Syst Bacteriol. 1983;33(4):772–6. doi: 10.1099/00207713-33-4-772 WOS:A1983RL79800012. - DOI
-
- Devriese LA, Hommez J, Wijfels R, Haesebrouck F. Composition of the enterococcal and streptococcal intestinal flora of poultry. J Appl Bacteriol. 1991;71(1):46–50. Epub 1991/07/01. . - PubMed
-
- Devriese LA, Cauwerts K, Hermans K, Wood AM. Enterococcus cecorum septicemia as a cause of bone and joint lesions resulting in lameness in broiler chickens. Vlaams Diergeneeskundig Tijdschrift. 2002;71(3):219–21. WOS:000176537600005.
MeSH terms
LinkOut - more resources
Full Text Sources

