Gain-of-function mutations in RPA1 cause a syndrome with short telomeres and somatic genetic rescue
- PMID: 34767620
- PMCID: PMC8854676
- DOI: 10.1182/blood.2021011980
Gain-of-function mutations in RPA1 cause a syndrome with short telomeres and somatic genetic rescue
Abstract
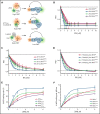
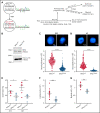
Human telomere biology disorders (TBD)/short telomere syndromes (STS) are heterogeneous disorders caused by inherited loss-of-function mutations in telomere-associated genes. Here, we identify 3 germline heterozygous missense variants in the RPA1 gene in 4 unrelated probands presenting with short telomeres and varying clinical features of TBD/STS, including bone marrow failure, myelodysplastic syndrome, T- and B-cell lymphopenia, pulmonary fibrosis, or skin manifestations. All variants cluster to DNA-binding domain A of RPA1 protein. RPA1 is a single-strand DNA-binding protein required for DNA replication and repair and involved in telomere maintenance. We showed that RPA1E240K and RPA1V227A proteins exhibit increased binding to single-strand and telomeric DNA, implying a gain in DNA-binding function, whereas RPA1T270A has binding properties similar to wild-type protein. To study the mutational effect in a cellular system, CRISPR/Cas9 was used to knock-in the RPA1E240K mutation into healthy inducible pluripotent stem cells. This resulted in severe telomere shortening and impaired hematopoietic differentiation. Furthermore, in patients with RPA1E240K, we discovered somatic genetic rescue in hematopoietic cells due to an acquired truncating cis RPA1 mutation or a uniparental isodisomy 17p with loss of mutant allele, coinciding with stabilized blood counts. Using single-cell sequencing, the 2 somatic genetic rescue events were proven to be independently acquired in hematopoietic stem cells. In summary, we describe the first human disease caused by germline RPA1 variants in individuals with TBD/STS.
© 2022 by The American Society of Hematology.
Figures



Comment in
-
Telomere biology disorders gain a family member.Blood. 2022 Feb 17;139(7):957-959. doi: 10.1182/blood.2021014533. Blood. 2022. PMID: 35175322 Free PMC article. No abstract available.
References
-
- Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193-1198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

