Iron homeostasis and organismal aging
- PMID: 34767974
- PMCID: PMC8620744
- DOI: 10.1016/j.arr.2021.101510
Iron homeostasis and organismal aging
Abstract
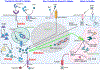
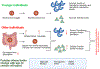
Iron is indispensable for normal body functions across species because of its critical roles in red blood cell function and many essential proteins and enzymes required for numerous physiological processes. Regulation of iron homeostasis is an intricate process involving multiple modulators at the systemic, cellular, and molecular levels. Interestingly, emerging evidence has demonstrated that many modulators of iron homeostasis contribute to organismal aging and longevity. On the other hand, the age-related dysregulation of iron homeostasis is often associated with multiple age-related pathologies including bone resorption and neurodegenerative diseases such as Alzheimer's disease. Thus, a thorough understanding on the interconnections between systemic and cellular iron balance and organismal aging may help decipher the etiologies of multiple age-related diseases, which could ultimately lead to developing therapeutic strategies to delay aging and treat various age-related diseases. Here we present the current understanding on the mechanisms of iron homeostasis. We also discuss the impacts of aging on iron homeostatic processes and how dysregulated iron metabolism may affect aging and organismal longevity.
Keywords: Aging; C. elegans; Drosophila; Homeostasis; Human diseases; Iron; Longevity.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no competing interests.
Figures


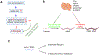

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

