Treatment of COVID-19 in pregnant women: A systematic review and meta-analysis
- PMID: 34768118
- PMCID: PMC8527829
- DOI: 10.1016/j.ejogrb.2021.10.007
Treatment of COVID-19 in pregnant women: A systematic review and meta-analysis
Abstract
Objective: Clinical trials evaluating pharmacological and non-pharmacological treatment of COVID-19, either excluded pregnant women or included very few women. Unlike the numerous systematic reviews on prevalence, symptoms and adverse outcomes of COVID-19 in pregnancy, there are very few on the effects of treatment on maternal and neonatal outcomes in pregnancy. We undertook a systematic review of all published and unpublished studies on the effects of pharmacological and non-pharmacological interventions for COVID-19 on maternal and neonatal pregnancy outcomes.
Data sources: We performed a systematic literature search of the following databases: Medline, Embase, Cochrane database, WHO (World Health Organization) COVID-19 database, China National Knowledge Infrastructure (CNKI), and Wanfang databases from 1 December 2019 to 1 December 2020.
Study eligibility criteria: Studies were only included if they involved pregnant or postnatal women who were exposed to pregnancy specific interventions like the mode of delivery and type of anaesthesia, pharmacological or non-pharmacological interventions.
Study appraisal and synthesis methods: We first screened the titles and abstracts of studies and then assessed the full text of the selected studies in detail for eligibility. Data on study design, population, type of screening for COVID-19, country, hospital, country status (high or low and middle income), treatment given (mode of delivery, type of anaesthesia, type of pharmacological and non-pharmacological treatment was extracted. The pre-defined maternal outcomes we collected were mode of delivery (vaginal or by caesarean section), severe or critical COVID-19 (as defined by the authors), symptomatic COVID-19, maternal death, maternal hospital admission, ICU admission, mechanical ventilation, ECMO and maternal pneumonia. The pre-defined neonatal outcomes we extracted were preterm birth (<37 weeks), stillbirth, neonatal death, NICU admission, neonatal COVID-19 positive, neonatal acidosis (pH < 7.0) and Apgar scores (<8 after 5 min). Study quality assessment was performed.
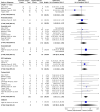
Results: From a total of 342 potential eligible studies, we included 27 studies in our systematic review, including 4943 pregnant women (appendix 3). Sixteen studies had a retrospective cohort design and 11 a prospective cohort design. There were no randomised controlled trials. There was a significant association between caesarean section and admission to ICU (OR 4.99, 95% CI 1.24 to 20.12; 4 studies, 153 women, I2 = 0%), and diagnosis of maternal COVID-19 pneumonia as defined by study authors (OR 3.09, 95% CI 1.52 to 6.28; 2 studies, 228 women, I2 = 0%). Women who had a preterm birth were more likely to have the baby via caesarean section (OR 3.03, 95% CI 1.71 to 5.36, 12 studies; 314 women, I2 = 0%). For pharmacological and non-pharmacological we provided estimates of the expected rates of outcomes in women exposed to various treatment of COVID-19. Comparative data for pregnant women, in particular for treatments proven to be effective in the general population, however, is lacking to provide clinically meaningful interpretation.
Conclusions: We found associations for pregnancy specific interventions, like mode of delivery and outcomes of the disease, but there were too few data on pharmacological and non-pharmacological treatments in pregnant women with COVID-19. We report the rates of complications found in the literature. We encourage researchers to include pregnant women in their trials and report the data on pregnant women separately.
Keywords: COVID-19; Meta-analysis; Neonatal; Pregnancy; Systematic review; Treatment.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Organization WH. Therapeutics and COVID-19: living guideline 2021 [Available from: https://apps.who.int/iris/bitstream/handle/10665/340374/WHO-2019-nCoVthe.... - PubMed
-
- Yap M., Debenham L., Kew T., Chatterjee S.R., Allotey J., Stallings E., et al. Clinical manifestations, prevalence, risk factors, outcomes, transmission, diagnosis and treatment of COVID-19 in pregnancy and postpartum: a living systematic review protocol. BMJ Open. 2020;10(12):e041868. doi: 10.1136/bmjopen-2020-041868. - DOI - PMC - PubMed
-
- Wells GA SB, O’Connell D, Peterson J, Welch V, Tugwell P, editor The NewcastleOttawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses [abstract]. 3rd Symposium on Systematic Reviews: Beyond the Basics; 2000; Oxford.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical