Prospective evaluation of ID NOW COVID-19 assay used as point-of-care test in an emergency department
- PMID: 34768231
- PMCID: PMC8556064
- DOI: 10.1016/j.jcv.2021.105021
Prospective evaluation of ID NOW COVID-19 assay used as point-of-care test in an emergency department
Abstract
Background: Rapid testing for COVID-19 has been clearly identified as an essential component of the strategy to control the SARS-CoV-2 epidemic, worldwide. The ID NOW COVID-19 assay is a simple, user-friendly, rapid molecular biology test based on nicking and extension amplification reaction (NEAR).
Objectives: The aim of this study was to evaluate the ID NOW COVID-19 assay when used as a point-of-care test (POCT) in our Emergency Department (ED).
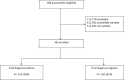
Type of study: This prospective study enrolled 395 consecutive patients; paired nasopharyngeal swabs were collected from each study participant. The first swab was tested with the ID NOW COVID-19 assay at the point-of-care by ED nurses. The second swab was diluted in viral transport medium (VTM) and sent to the clinical microbiology department for analysis by both the RT-PCR Simplexa test COVID-19 Direct assay as the study reference method, and the ID NOW COVID-19 assay performed in the laboratory.
Results: Nasopharyngeal swabs directly tested with the ID NOW COVID-19 assay yielded a sensitivity, specificity, PPV and NPV of 98.0%, 97.5%, 96.2% and 98.7%, respectively, in comparison with the RT-PCR study reference assay. When the ID NOW COVID-19 assay was performed in the laboratory using the VTM samples, the sensitivity decreased to 62.5% and the NPV to 79.7%. Three false negative test results were reported with the ID NOW COVID-19 assay when performed using undiluted swabs directly in the ED; these results were obtained from patients with elevated CT values (> 30).
Conclusion: We demonstrated that the ID NOW COVID-19 assay, performed as a point of care test in the ED using dry swabs, provides a rapid and reliable alternative to laboratory-based RT-PCR methods.
Keywords: COVID-19; Emergency department; ID NOW COVID-19; Isothermal amplification; Point-of-care test; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
We have no conflicts of interest to declare.
Figures

References
-
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. China novel coronavirus investigating and research team, a novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Emery S.L., Erdman D.D., Bowen M.D., Newton B.R., Winchell J.M., Meyer R.F., Tong S., Cook B.T., Holloway B.P., McCaustland K.A., Rota P.A., Bankamp B., Lowe L.E., Ksiazek T.G., Bellini W.J., Anderson L.J. Real-time reverse transcription-polymerase chain reaction assay for SARS-associated coronavirus. Emerg. Infect. Dis. 2004;10:311–316. doi: 10.3201/eid1002.030759. - DOI - PMC - PubMed
-
- Abbott Laboratories . Abbott Labora-tories; Chicago, IL: 2000. ID Now COVID-19 Package Insert.https://www.fda.gov/media/136525/download (n.d.)
-
- Harrington A., Cox B., Snowdon J., Bakst J., Ley E., Grajales P., Maggiore J., Kahn S. Comparison of Abbott ID now and Abbott m2000 methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal swabs from symptomatic patients. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00798-20. e00798-20, /jcm/58/8/JCM.00798-20.atom. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

