Femoral µCT Analysis, Mechanical Testing and Immunolocalization of Bone Proteins in β-Hydroxy β-Methylbutyrate (HMB) Supplemented Spiny Mouse in a Model of Pregnancy and Lactation-Associated Osteoporosis
- PMID: 34768327
- PMCID: PMC8584851
- DOI: 10.3390/jcm10214808
Femoral µCT Analysis, Mechanical Testing and Immunolocalization of Bone Proteins in β-Hydroxy β-Methylbutyrate (HMB) Supplemented Spiny Mouse in a Model of Pregnancy and Lactation-Associated Osteoporosis
Abstract
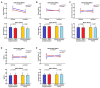
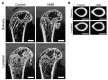
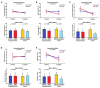
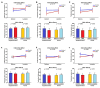
A metabolite of leucine, ß-hydroxy-ß-methylbutyrate (HMB), used as a dietary supplement effects muscle tissue gain and bone tissue quality. Since there are no studies on the effects of HMB during pregnancy yet, the aim of the current study was to determine the effects of HMB supplementation during pregnancy on osteoporotic bone quality postpartum and post-lactation using spiny mice (Acomys cahirinus) as the animal models. The six-month-old dams were divided into four groups: pregnant and lactating controls, and pregnant and lactating HMB-treated (during the second trimester of pregnancy) females. The intensity of the immunoreaction of osteocalcin (OC), osteoprotegerin (OPG), bone morphogenetic protein 2 (BMP-2), tissue inhibitor of metalloproteinases 2 (TIMP-2), matrix metalloproteinase 8 and 13 (MMP-8 and MMP-13) and proteins involved in bone turnover, was measured in femoral trabecular and compact bone, as well as in the hyaline and epiphyseal cartilage of the femora. The analysis of the trabecular bone microarchitecture showed that the administration of HMB to pregnant females, by influencing the proteins responsible for bone cell activity and collagen remodeling, can provide protection from bone loss. Based on the results of the current study it can be assumed that HMB administration to pregnant females has a more positive impact on trabecular than compact bone.
Keywords: bone; bone proteins; bone quality; lactation; pregnancy; β-hydroxy β-methylbutyrate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
β-Hydroxy-β-Methylbutyrate (HMB) Supplementation Prevents Bone Loss during Pregnancy-Novel Evidence from a Spiny Mouse (Acomys cahirinus) Model.Int J Mol Sci. 2021 Mar 17;22(6):3047. doi: 10.3390/ijms22063047. Int J Mol Sci. 2021. PMID: 33802646 Free PMC article.
-
Maternal HMB treatment affects bone and hyaline cartilage development in their weaned piglets via the leptin/osteoprotegerin system.J Anim Physiol Anim Nutr (Berl). 2019 Mar;103(2):626-643. doi: 10.1111/jpn.13060. Epub 2019 Jan 18. J Anim Physiol Anim Nutr (Berl). 2019. PMID: 30659706
-
The effect of maternal HMB supplementation on bone mechanical and geometrical properties, as well as histomorphometry and immunolocalization of VEGF, TIMP2, MMP13, BMP2 in the bone and cartilage tissue of the humerus of their newborn piglets.PLoS One. 2021 Feb 24;16(2):e0240642. doi: 10.1371/journal.pone.0240642. eCollection 2021. PLoS One. 2021. PMID: 33626093 Free PMC article.
-
[β-hydroxy-β-methylbutyrate as a dietary supplement (I): metabolism and toxicity].Nutr Hosp. 2014 Nov 27;31(2):590-6. doi: 10.3305/nh.2015.31.2.8432. Nutr Hosp. 2014. PMID: 25617539 Review. Spanish.
-
Effects of Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Elderly Body Composition and Muscle Strength: A Review of Clinical Trials.Ann Nutr Metab. 2021;77(1):16-22. doi: 10.1159/000514236. Epub 2021 Mar 12. Ann Nutr Metab. 2021. PMID: 33709969 Review.
Cited by
-
Bioinformatics analysis of key genes, immune infiltration, and risk assessment in low bone mineral density among perimenopausal women: An observational study.Medicine (Baltimore). 2024 Jul 5;103(27):e38695. doi: 10.1097/MD.0000000000038695. Medicine (Baltimore). 2024. PMID: 38968517 Free PMC article.
-
ß-Hydroxy-ß-methylbutyrate: A feed supplement influencing performance, bone metabolism, intestinal morphology, and muscle quality of laying hens: a preliminary one-point study.Poult Sci. 2024 May;103(5):103597. doi: 10.1016/j.psj.2024.103597. Epub 2024 Feb 28. Poult Sci. 2024. PMID: 38471225 Free PMC article.
-
Beyond Calcium and Vitamin D: Exploring Creatine, β-Hydroxy-β-methylbutyrate, Prebiotics and Probiotics in Osteosarcopenia.Nutrients. 2025 Jul 16;17(14):2332. doi: 10.3390/nu17142332. Nutrients. 2025. PMID: 40732957 Free PMC article. Review.
References
-
- Rabijewski M. Risk factors for osteoporosis, with particular emphasis on the correct development and metabolism of bone tissue. Inf. Forum. 2017;8:77–82.
-
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy Osteoporosis, prevention and treatment. J. Am. Med. Assoc. 2001;285:765–795.
-
- Kovacs C.S., Kronenberg H.M. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr. Rev. 1997;18:832–872. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

