Prognostic Value of Sarcopenia in Metastatic Colorectal Cancer Patients Treated with Trifluridine/Tipiracil
- PMID: 34768626
- PMCID: PMC8584514
- DOI: 10.3390/jcm10215107
Prognostic Value of Sarcopenia in Metastatic Colorectal Cancer Patients Treated with Trifluridine/Tipiracil
Abstract
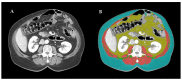
Sarcopenia is common in metastatic colorectal cancer (mCRC), increases the risk of treatment-related toxicity and reduces survival. Trifluridine/tipiracil (TT) chemotherapy significantly improved survival in refractory mCRC patients, but the prognostic and predictive role of pretherapeutic sarcopenia and variation in the skeletal muscle index (SMI) during this treatment has not been investigated so far. In this retrospective, observational study, clinical data on mCRC patients treated with TT at six cancer centres in Poland were collected. Computed tomography (CT) scans acquired at the time of initiation of TT (CT1) and on the first restaging (CT2), were evaluated. SMI was assessed based on the skeletal muscle area (SMA) at the level of the third lumbar vertebra. Progression-free survival (PFS) and overall survival (OS) were calculated from the treatment start. Neither initial sarcopenia nor ≥5% skeletal mass loss (SML) between CT1 and CT2 had a significant effect on PFS in treated patients (p = 0.5526 and p = 0.1092, respectively). In the multivariate analysis, reduced OS was found in patients with ≥5% SML (HR: 2.03 (1.11-3.72), p = 0.0039). We describe the prognostic role of sarcopenia beyond second line treatment and analyze other factors, such as performance status, tumor histological differentiation or carcinoembryonic antigen level that could predict TT treatment response.
Keywords: cancer cachexia; metastatic colorectal cancer; sarcopenia; trifluridine/tipiracil.
Conflict of interest statement
M. Malik: Travel/Accommodation/Expenses: Servier, Bristol-Myers Squibb. M. Gełej: Honoraria (self): Servier; B. Kania-Zembaczyńska: Honoraria (self): Servier, Amgen, Merck. L. Bodnar: Advisory/Consultancy: GSK Commercial Sp z o.o, Speaker Bureau/Expert testimony: Roche, Ipsen, Amgen; Travel/Accommodation/Expenses: Servier. All other authors have declared no conflicts of interest.
Figures


References
-
- Krzakowski M., Potemski P., Warzocha K., Wysocki P. Onkologia Kliniczna. Via Medica; Gdańsk, Poland: 2015.
-
- Warren S. The Immediate Causes of Death in Cancer. Am. J. Med. Sci. 1932;184:610–615. doi: 10.1097/00000441-193211000-00002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

