Prion Infectivity and PrPBSE in the Peripheral and Central Nervous System of Cattle 8 Months Post Oral BSE Challenge
- PMID: 34768738
- PMCID: PMC8583047
- DOI: 10.3390/ijms222111310
Prion Infectivity and PrPBSE in the Peripheral and Central Nervous System of Cattle 8 Months Post Oral BSE Challenge
Abstract
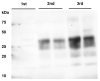
After oral exposure of cattle with classical bovine spongiform encephalopathy (C-BSE), the infectious agent ascends from the gut to the central nervous system (CNS) primarily via the autonomic nervous system. However, the timeline of this progression has thus far remained widely undetermined. Previous studies were focused on later time points after oral exposure of animals that were already 4 to 6 months old when challenged. In contrast, in this present study, we have orally inoculated 4 to 6 weeks old unweaned calves with high doses of BSE to identify any possible BSE infectivity and/or PrPBSE in peripheral nervous tissues during the first eight months post-inoculation (mpi). For the detection of BSE infectivity, we used a bovine PrP transgenic mouse bioassay, while PrPBSE depositions were analyzed by immunohistochemistry (IHC) and by protein misfolding cyclic amplification (PMCA). We were able to show that as early as 8 mpi the thoracic spinal cord as well as the parasympathetic nodal ganglion of these animals contained PrPBSE and BSE infectivity. This shows that the centripetal prion spread starts early after challenge at least in this age group, which represents an essential piece of information for the risk assessments for food, feed, and pharmaceutical products produced from young calves.
Keywords: BSE; PrPBSE; cattle; infectivity; peripheral and central nervous system; prion protein; protein misfolding cyclic amplification (PMCA).
Conflict of interest statement
The authors declare no conflict of interest. Parts of this manuscript have been published in a dissertation (Ackermann 2018, University of Veterinary Medicine Hannover).
Figures


Similar articles
-
Detection of PrPBSE and prion infectivity in the ileal Peyer's patch of young calves as early as 2 months after oral challenge with classical bovine spongiform encephalopathy.Vet Res. 2017 Dec 19;48(1):88. doi: 10.1186/s13567-017-0495-5. Vet Res. 2017. PMID: 29258602 Free PMC article.
-
Detection of PrPres in peripheral tissue in pigs with clinical disease induced by intracerebral challenge with sheep-passaged bovine spongiform encephalopathy agent.PLoS One. 2018 Jul 5;13(7):e0199914. doi: 10.1371/journal.pone.0199914. eCollection 2018. PLoS One. 2018. PMID: 29975760 Free PMC article.
-
Spread of classic BSE prions from the gut via the peripheral nervous system to the brain.Am J Pathol. 2012 Aug;181(2):515-24. doi: 10.1016/j.ajpath.2012.05.001. Epub 2012 Jul 9. Am J Pathol. 2012. PMID: 22781833
-
Transgenic models of prion disease.Arch Virol Suppl. 2000;(16):113-24. doi: 10.1007/978-3-7091-6308-5_10. Arch Virol Suppl. 2000. PMID: 11214913 Review.
-
Pathogenesis of classical and atypical BSE in cattle.Prev Vet Med. 2011 Nov 1;102(2):112-7. doi: 10.1016/j.prevetmed.2011.04.006. Epub 2011 May 17. Prev Vet Med. 2011. PMID: 21592603 Review.
Cited by
-
Bovine spongiform encephalopathy: A review of current knowledge and challenges.Open Vet J. 2025 Jan;15(1):54-68. doi: 10.5455/OVJ.2025.v15.i1.5. Epub 2025 Jan 31. Open Vet J. 2025. PMID: 40092198 Free PMC article. Review.
-
Transport of Prions in the Peripheral Nervous System: Pathways, Cell Types, and Mechanisms.Viruses. 2022 Mar 18;14(3):630. doi: 10.3390/v14030630. Viruses. 2022. PMID: 35337037 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

