BDNF-Overexpressing Engineered Mesenchymal Stem Cells Enhances Their Therapeutic Efficacy against Severe Neonatal Hypoxic Ischemic Brain Injury
- PMID: 34768827
- PMCID: PMC8583727
- DOI: 10.3390/ijms222111395
BDNF-Overexpressing Engineered Mesenchymal Stem Cells Enhances Their Therapeutic Efficacy against Severe Neonatal Hypoxic Ischemic Brain Injury
Abstract
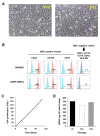
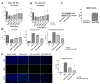
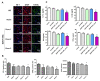
We investigated whether irradiated brain-derived neurotropic factor (BDNF)-overexpressing engineered human mesenchymal stem cells (BDNF-eMSCs) improve paracrine efficiency and, thus, the beneficial potency of naïve MSCs against severe hypoxic ischemic (HI) brain injury in newborn rats. Irradiated BDNF-eMSCs hyper-secreted BDNF > 10 fold and were >5 fold more effective than naïve MSCs in attenuating the oxygen-glucose deprivation-induced increase in cytotoxicity, oxidative stress, and cell death in vitro. Only the irradiated BDNF-eMSCs, but not naïve MSCs, showed significant attenuating effects on severe neonatal HI-induced short-term brain injury scores, long-term progress of brain infarct, increased apoptotic cell death, astrogliosis and inflammatory responses, and impaired negative geotaxis and rotarod tests in vivo. Our data, showing better paracrine potency and the resultant better therapeutic efficacy of the irradiated BDNF-eMSCs, compared to naïve MSCs, suggest that MSCs transfected with the BDNF gene might represent a better, new therapeutic strategy against severe neonatal HI brain injury.
Keywords: brain; brain derived neurotropic factor; cell transplantation; hypoxia-ischemia; infant; mesenchymal stem cell transplantation; newborn; stem cells.
Conflict of interest statement
The authors declare no conflict of interest, except for H.-J.K. and S.M.L. Hyo-Jin Kim and Soon Min Lee are salaried employees of SL BiGen, Inc.
Figures

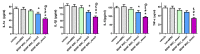


Similar articles
-
Thrombin Preconditioning Enhances Therapeutic Efficacy of Human Wharton's Jelly-Derived Mesenchymal Stem Cells in Severe Neonatal Hypoxic Ischemic Encephalopathy.Int J Mol Sci. 2019 May 20;20(10):2477. doi: 10.3390/ijms20102477. Int J Mol Sci. 2019. PMID: 31137455 Free PMC article.
-
Human pluripotent stem cell-derived ectomesenchymal stromal cells promote more robust functional recovery than umbilical cord-derived mesenchymal stromal cells after hypoxic-ischaemic brain damage.Theranostics. 2022 Jan 1;12(1):143-166. doi: 10.7150/thno.57234. eCollection 2022. Theranostics. 2022. PMID: 34987639 Free PMC article.
-
Pivotal Role of Brain-Derived Neurotrophic Factor Secreted by Mesenchymal Stem Cells in Severe Intraventricular Hemorrhage in Newborn Rats.Cell Transplant. 2017 Jan 24;26(1):145-156. doi: 10.3727/096368916X692861. Epub 2016 Aug 16. Cell Transplant. 2017. PMID: 27535166 Free PMC article.
-
Umbilical cord blood mesenchymal stem cells co-modified by TERT and BDNF: a novel neuroprotective therapy for neonatal hypoxic-ischemic brain damage.Int J Dev Neurosci. 2014 Nov;38:147-54. doi: 10.1016/j.ijdevneu.2014.06.014. Epub 2014 Jul 3. Int J Dev Neurosci. 2014. PMID: 24999119 Review.
-
Stem cells for neonatal brain injury - Lessons from the bench.Semin Perinatol. 2023 Apr;47(3):151726. doi: 10.1016/j.semperi.2023.151726. Epub 2023 Mar 12. Semin Perinatol. 2023. PMID: 37003920 Review.
Cited by
-
MSC based gene delivery methods and strategies improve the therapeutic efficacy of neurological diseases.Bioact Mater. 2022 Nov 30;23:409-437. doi: 10.1016/j.bioactmat.2022.11.007. eCollection 2023 May. Bioact Mater. 2022. PMID: 36474656 Free PMC article. Review.
-
MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents-A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives.Int J Mol Sci. 2022 Mar 2;23(5):2745. doi: 10.3390/ijms23052745. Int J Mol Sci. 2022. PMID: 35269887 Free PMC article. Review.
-
Progress in Research on Stem Cells in Neonatal Refractory Diseases.J Pers Med. 2023 Aug 21;13(8):1281. doi: 10.3390/jpm13081281. J Pers Med. 2023. PMID: 37623531 Free PMC article. Review.
-
Combination of induced pluripotent stem cell-derived motor neuron progenitor cells with irradiated brain-derived neurotrophic factor over-expressing engineered mesenchymal stem cells enhanced restoration of axonal regeneration in a chronic spinal cord injury rat model.Stem Cell Res Ther. 2024 Jun 18;15(1):173. doi: 10.1186/s13287-024-03770-9. Stem Cell Res Ther. 2024. PMID: 38886817 Free PMC article.
-
Improving the future of clinical trials and translation of mesenchymal stromal cell therapies for neonatal disorders.Stem Cells Transl Med. 2024 Oct 10;13(10):941-948. doi: 10.1093/stcltm/szae060. Stem Cells Transl Med. 2024. PMID: 39120439 Free PMC article. Review.
References
-
- Shankaran S., Laptook A., Wright L.L., Ehrenkranz R.A., Donovan E.F., Fanaroff A.A., Stark A.R., Tyson J.E., Poole K., Carl W.A., et al. Whole-Body Hypothermia for Neonatal Encephalopathy: Animal Observations as a Basis for a Randomized, Controlled Pilot Study in Term Infants. Pediatrics. 2002;110:377–385. doi: 10.1542/peds.110.2.377. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

