Saikosaponin A and D Inhibit Adipogenesis via the AMPK and MAPK Signaling Pathways in 3T3-L1 Adipocytes
- PMID: 34768840
- PMCID: PMC8583978
- DOI: 10.3390/ijms222111409
Saikosaponin A and D Inhibit Adipogenesis via the AMPK and MAPK Signaling Pathways in 3T3-L1 Adipocytes
Abstract
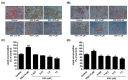
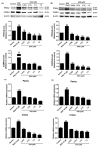
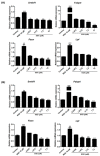
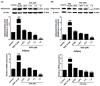
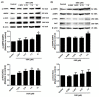
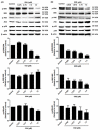
Obesity is a lipid metabolism disorder caused by genetic, medicinal, nutritional, and other environmental factors. It is characterized by a complex condition of excess lipid accumulation in adipocytes. Adipogenesis is a differentiation process that converts preadipocytes into mature adipocytes and contributes to excessive fat deposition. Saikosaponin A (SSA) and saikosaponin D (SSD) are triterpenoid saponins separated from the root of the Bupleurum chinensis, which has long been used to treat inflammation, fever, and liver diseases. However, the effects of these constituents on lipid accumulation and obesity are poorly understood. We investigated the anti-obesity effects of SSA and SSD in mouse 3T3-L1 adipocytes. The MTT assay was performed to measure cell viability, and Oil Red O staining was conducted to determine lipid accumulation. Various adipogenic transcription factors were evaluated at the protein and mRNA levels by Western blot assay and quantitative reverse transcription polymerase chain reaction (qRT-PCR). Here, we showed that SSA and SSD significantly inhibited lipid accumulation without affecting cell viability within the range of the tested concentrations (0.938-15 µM). SSA and SSD also dose-dependently suppressed the expression of peroxisome proliferator-activated receptor gamma (PPARγ), CCAAT/enhancer binding protein alpha (C/EBPα), sterol regulatory element binding protein-1c (SREBP-1c), and adiponectin. Furthermore, the decrease of these transcriptional factors resulted in the repressed expression of several lipogenic genes including fatty acid binding protein (FABP4), fatty acid synthase (FAS), and lipoprotein lipase (LPL). In addition, SSA and SSD enhanced the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and its substrate, acetyl-CoA carboxylase (ACC), and inhibited the phosphorylation of extracellular-regulated kinase 1/2 (ERK1/2) and p38, but not c-Jun-N-terminal kinase (JNK). These results suggest that SSA and SSD inhibit adipogenesis through the AMPK or mitogen-activated protein kinase (MAPK) pathways in the early stages of adipocyte differentiation. This is the first study on the anti-adipogenic effects of SSA and SSD, and further research in animals and humans is necessary to confirm the potential of saikosaponins as therapeutic agents for obesity.
Keywords: 3T3-L1 cells; AMPK pathway; MAPK pathway; adipogenesis; saikosaponin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

