Overexpression of lipA or glpD_RuBisCO in the Synechocystis sp. PCC 6803 Mutant Lacking the Aas Gene Enhances Free Fatty-Acid Secretion and Intracellular Lipid Accumulation
- PMID: 34768898
- PMCID: PMC8583886
- DOI: 10.3390/ijms222111468
Overexpression of lipA or glpD_RuBisCO in the Synechocystis sp. PCC 6803 Mutant Lacking the Aas Gene Enhances Free Fatty-Acid Secretion and Intracellular Lipid Accumulation
Abstract
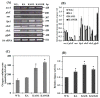
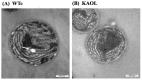
Although engineered cyanobacteria for the production of lipids and fatty acids (FAs) are intelligently used as sustainable biofuel resources, intracellularly overproduced FAs disturb cellular homeostasis and eventually generate lethal toxicity. In order to improve their production by enhancing FFAs secretion into a medium, we constructed three engineered Synechocystis 6803 strains including KA (a mutant lacking the aas gene), KAOL (KA overexpressing lipA, encoding lipase A in membrane lipid hydrolysis), and KAOGR (KA overexpressing quadruple glpD/rbcLXS, related to the CBB cycle). Certain contents of intracellular lipids and secreted FFAs of all engineered strains were higher than those of the wild type. Remarkably, the KAOL strain attained the highest level of secreted FFAs by about 21.9%w/DCW at day 5 of normal BG11 cultivation, with a higher growth rate and shorter doubling time. TEM images provided crucial evidence on the morphological changes of the KAOL strain, which accumulated abundant droplets on regions of thylakoid membranes throughout the cell when compared with wild type. On the other hand, BG11-N condition significantly induced contents of both intracellular lipids and secreted FFAs of the KAOL strain up to 37.2 and 24.5%w/DCW, respectively, within 5 days. Then, for the first time, we shone a spotlight onto the overexpression of lipA in the aas mutant of Synechocystis as another potential strategy to achieve higher FFAs secretion with sustainable growth.
Keywords: FFA secretion; Synechocystis sp. PCC 6803; acyl-ACP synthetase; lipase A; membrane lipid degradation.
Conflict of interest statement
None of the authors have any financial conflict of interest that might be constructed to influence the results or interpretation of this manuscript.
Figures







Similar articles
-
Enhanced productivity of extracellular free fatty acids by gene disruptions of acyl-ACP synthetase and S-layer protein in Synechocystis sp. PCC 6803.Biotechnol Biofuels Bioprod. 2022 Sep 24;15(1):99. doi: 10.1186/s13068-022-02197-9. Biotechnol Biofuels Bioprod. 2022. PMID: 36153604 Free PMC article.
-
Synechocystis sp. PCC 6803 overexpressing genes involved in CBB cycle and free fatty acid cycling enhances the significant levels of intracellular lipids and secreted free fatty acids.Sci Rep. 2020 Mar 11;10(1):4515. doi: 10.1038/s41598-020-61100-4. Sci Rep. 2020. PMID: 32161307 Free PMC article.
-
High-Light-Induced Stress Activates Lipid Deacylation at the Sn-2 Position in the Cyanobacterium Synechocystis Sp. PCC 6803.Plant Cell Physiol. 2022 Jan 25;63(1):82-91. doi: 10.1093/pcp/pcab147. Plant Cell Physiol. 2022. PMID: 34623441 Free PMC article.
-
Improved lipid production via fatty acid biosynthesis and free fatty acid recycling in engineered Synechocystis sp. PCC 6803.Biotechnol Biofuels. 2019 Jan 4;12:8. doi: 10.1186/s13068-018-1349-8. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 30622650 Free PMC article.
-
Current knowledge and recent advances in understanding metabolism of the model cyanobacterium Synechocystis sp. PCC 6803.Biosci Rep. 2020 Apr 30;40(4):BSR20193325. doi: 10.1042/BSR20193325. Biosci Rep. 2020. PMID: 32149336 Free PMC article. Review.
Cited by
-
The adc1 knockout with proC overexpression in Synechocystis sp. PCC 6803 induces a diversion of acetyl-CoA to produce more polyhydroxybutyrate.Biotechnol Biofuels Bioprod. 2024 Jan 13;17(1):6. doi: 10.1186/s13068-024-02458-9. Biotechnol Biofuels Bioprod. 2024. PMID: 38218963 Free PMC article.
-
Increased Biomass and Polyhydroxybutyrate Production by Synechocystis sp. PCC 6803 Overexpressing RuBisCO Genes.Int J Mol Sci. 2023 Mar 29;24(7):6415. doi: 10.3390/ijms24076415. Int J Mol Sci. 2023. PMID: 37047389 Free PMC article.
-
Insufficient Acetyl-CoA Pool Restricts the Phototrophic Production of Organic Acids in Model Cyanobacteria.Int J Mol Sci. 2024 Nov 1;25(21):11769. doi: 10.3390/ijms252111769. Int J Mol Sci. 2024. PMID: 39519321 Free PMC article. Review.
-
Enhanced productivity of extracellular free fatty acids by gene disruptions of acyl-ACP synthetase and S-layer protein in Synechocystis sp. PCC 6803.Biotechnol Biofuels Bioprod. 2022 Sep 24;15(1):99. doi: 10.1186/s13068-022-02197-9. Biotechnol Biofuels Bioprod. 2022. PMID: 36153604 Free PMC article.
-
Improved lipid production and component of mycosporine-like amino acids by co-overexpression of amt1 and aroB genes in Synechocystis sp. PCC6803.Sci Rep. 2023 Nov 9;13(1):19439. doi: 10.1038/s41598-023-46290-x. Sci Rep. 2023. PMID: 37945676 Free PMC article.
References
-
- Huntley M.E., Redalje D.G. CO2 mitigation and renewable oil from photosynthetic microbes: A new appraisal. Mitig. Adapt. Strateg. Glob. Chang. 2007;12:573–608. doi: 10.1007/s11027-006-7304-1. - DOI
-
- Eungrasamee K., Incharoensakdi A., Lindblad P., Jantaro S. Synechocystis sp. PCC 6803 overexpressing genes involved in CBB cycle and free fatty acid cycling enhances the significant levels of intracellular lipids and secreted free fatty acids. Sci. Rep. 2020;10:4515. doi: 10.1038/s41598-020-61100-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

