Metabolic Reprogramming in COVID-19
- PMID: 34768906
- PMCID: PMC8584248
- DOI: 10.3390/ijms222111475
Metabolic Reprogramming in COVID-19
Abstract
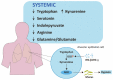
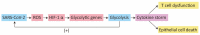
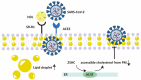
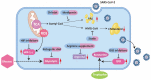
Plenty of research has revealed virus induced alternations in metabolic pathways, which is known as metabolic reprogramming. Studies focusing on COVID-19 have uncovered significant changes in metabolism, resulting in the perspective that COVID-19 is a metabolic disease. Reprogramming of amino acid, glucose, cholesterol and fatty acid is distinctive characteristic of COVID-19 infection. These metabolic changes in COVID-19 have a critical role not only in producing energy and virus constituent elements, but also in regulating immune response, offering new insights into COVID-19 pathophysiology. Remarkably, metabolic reprogramming provides great opportunities for developing novel biomarkers and therapeutic agents for COVID-19 infection. Such novel agents are expected to be effective adjuvant therapies. In this review, we integrate present studies about major metabolic reprogramming in COVID-19, as well as the possibility of targeting reprogrammed metabolism to combat virus infection.
Keywords: COVID-19; arginine; cholesterol; fatty acids; glucose; metabolic changes; tryptophan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression.Cell Mol Life Sci. 2016 Jan;73(2):377-92. doi: 10.1007/s00018-015-2070-4. Epub 2015 Oct 23. Cell Mol Life Sci. 2016. PMID: 26499846 Free PMC article. Review.
-
Metabolic Reprogramming and Potential Therapeutic Targets in Lymphoma.Int J Mol Sci. 2023 Mar 13;24(6):5493. doi: 10.3390/ijms24065493. Int J Mol Sci. 2023. PMID: 36982568 Free PMC article. Review.
-
Metabolomics and Metabolic Reprogramming in Kidney Cancer.Semin Nephrol. 2018 Mar;38(2):175-182. doi: 10.1016/j.semnephrol.2018.01.006. Semin Nephrol. 2018. PMID: 29602399 Free PMC article. Review.
-
Exploring metabolic reprogramming in esophageal cancer: the role of key enzymes in glucose, amino acid, and nucleotide pathways and targeted therapies.Cancer Gene Ther. 2025 Feb;32(2):165-183. doi: 10.1038/s41417-024-00858-5. Epub 2025 Jan 10. Cancer Gene Ther. 2025. PMID: 39794467 Review.
-
Metabolic reprogramming in clear cell renal cell carcinoma.Nat Rev Nephrol. 2017 Jul;13(7):410-419. doi: 10.1038/nrneph.2017.59. Epub 2017 May 8. Nat Rev Nephrol. 2017. PMID: 28480903 Review.
Cited by
-
Salivary Metabolomic Analysis Reveals Amino Acid Metabolism Shift in SARS-CoV-2 Virus Activity and Post-Infection Condition.Metabolites. 2023 Feb 11;13(2):263. doi: 10.3390/metabo13020263. Metabolites. 2023. PMID: 36837882 Free PMC article.
-
PKM2 induces mitophagy through the AMPK-mTOR pathway promoting CSFV proliferation.J Virol. 2024 Mar 19;98(3):e0175123. doi: 10.1128/jvi.01751-23. Epub 2024 Feb 6. J Virol. 2024. PMID: 38319105 Free PMC article.
-
Unbalanced IDO1/IDO2 Endothelial Expression and Skewed Keynurenine Pathway in the Pathogenesis of COVID-19 and Post-COVID-19 Pneumonia.Biomedicines. 2022 Jun 6;10(6):1332. doi: 10.3390/biomedicines10061332. Biomedicines. 2022. PMID: 35740354 Free PMC article. Review.
-
Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID.NPJ Sci Food. 2024 Mar 30;8(1):19. doi: 10.1038/s41538-024-00261-2. NPJ Sci Food. 2024. PMID: 38555403 Free PMC article. Review.
-
Amino Acid Metabolism in Leukocytes Showing In Vitro IgG Memory from SARS-CoV2-Infected Patients.Diseases. 2024 Feb 23;12(3):43. doi: 10.3390/diseases12030043. Diseases. 2024. PMID: 38534967 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

