Pathophysiology and Molecular Imaging of Diabetic Foot Infections
- PMID: 34768982
- PMCID: PMC8584017
- DOI: 10.3390/ijms222111552
Pathophysiology and Molecular Imaging of Diabetic Foot Infections
Abstract
Diabetic foot infection is the leading cause of non-traumatic lower limb amputations worldwide. In addition, diabetes mellitus and sequela of the disease are increasing in prevalence. In 2017, 9.4% of Americans were diagnosed with diabetes mellitus (DM). The growing pervasiveness and financial implications of diabetic foot infection (DFI) indicate an acute need for improved clinical assessment and treatment. Complex pathophysiology and suboptimal specificity of current non-invasive imaging modalities have made diagnosis and treatment response challenging. Current anatomical and molecular clinical imaging strategies have mainly targeted the host's immune responses rather than the unique metabolism of the invading microorganism. Advances in imaging have the potential to reduce the impact of these problems and improve the assessment of DFI, particularly in distinguishing infection of soft tissue alone from osteomyelitis (OM). This review presents a summary of the known pathophysiology of DFI, the molecular basis of current and emerging diagnostic imaging techniques, and the mechanistic links of these imaging techniques to the pathophysiology of diabetic foot infections.
Keywords: DWI; SPECT; X-ray; diabetic foot infection; molecular imaging; optical tomography; test predictive value.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
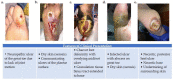

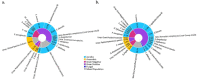
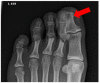
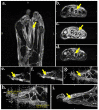




References
-
- Centers for Disease Control and Prevention National Diabetes Statistics Report. [(accessed on 8 October 2019)];2017 Available online: cdc.gov/media/releases/2017/p0718-diabetes-report.html.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

