Not All Lectins Are Equally Suitable for Labeling Rodent Vasculature
- PMID: 34768985
- PMCID: PMC8584019
- DOI: 10.3390/ijms222111554
Not All Lectins Are Equally Suitable for Labeling Rodent Vasculature
Abstract
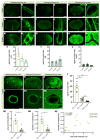
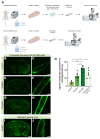
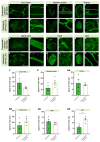
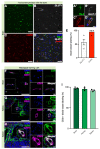
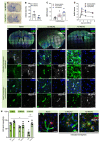
The vascular system is vital for all tissues and the interest in its visualization spans many fields. A number of different plant-derived lectins are used for detection of vasculature; however, studies performing direct comparison of the labeling efficacy of different lectins and techniques are lacking. In this study, we compared the labeling efficacy of three lectins: Griffonia simplicifolia isolectin B4 (IB4); wheat germ agglutinin (WGA), and Lycopersicon esculentum agglutinin (LEA). The LEA lectin was identified as being far superior to the IB4 and WGA lectins in histological labeling of blood vessels in brain sections. A similar signal-to-noise ratio was achieved with high concentrations of the WGA lectin injected during intracardial perfusion. Lectins were also suitable for labeling vasculature in other tissues, including spinal cord, dura mater, heart, skeletal muscle, kidney, and liver tissues. In uninjured tissues, the LEA lectin was as accurate as the Tie2-eGFP reporter mice and GLUT-1 immunohistochemistry for labeling the cerebral vasculature, validating its specificity and sensitivity. However, in pathological situations, e.g., in stroke, the sensitivity of the LEA lectin decreases dramatically, limiting its applicability in such studies. This work can be used for selecting the type of lectin and labeling method for various tissues.
Keywords: angiogenesis; blood vessels; lectin angiography; lectins; stroke; vascular research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

