Targeting Folate Metabolism Is Selectively Cytotoxic to Glioma Stem Cells and Effectively Cooperates with Differentiation Therapy to Eliminate Tumor-Initiating Cells in Glioma Xenografts
- PMID: 34769063
- PMCID: PMC8583947
- DOI: 10.3390/ijms222111633
Targeting Folate Metabolism Is Selectively Cytotoxic to Glioma Stem Cells and Effectively Cooperates with Differentiation Therapy to Eliminate Tumor-Initiating Cells in Glioma Xenografts
Abstract
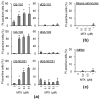
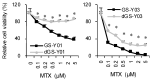
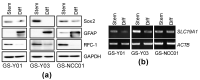
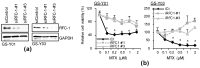
Glioblastoma (GBM) is one of the deadliest of all human cancers. Developing therapies targeting GBM cancer stem cells or glioma stem cells (GSCs), which are deemed responsible for the malignancy of GBM due to their therapy resistance and tumor-initiating capacity, is considered key to improving the dismal prognosis of GBM patients. In this study, we found that folate antagonists, such as methotrexate (MTX) and pemetrexed, are selectively cytotoxic to GSCs, but not to their differentiated counterparts, normal fibroblasts, or neural stem cells in vitro, and that the high sensitivity of GCSs to anti-folates may be due to the increased expression of RFC-1/SLC19A1, the reduced folate carrier that transports MTX into cells, in GSCs. Of note, in an in vivo serial transplantation model, MTX alone failed to exhibit anti-GSC effects but promoted the anti-GSC effects of CEP1347, an inducer of GSC differentiation. This suggests that folate metabolism, which plays an essential role specifically in GSCs, is a promising target of anti-GSC therapy, and that the combination of cytotoxic and differentiation therapies may be a novel and promising approach to effectively eliminate cancer stem cells.
Keywords: JNK; RFC-1; anti-folate; brain tumor initiating cells; glioma stem cell; serial transplantation assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Okada M., Takeda H., Sakaki H., Kuramoto K., Suzuki S., Sanomachi T., Togashi K., Seino S., Kitanaka C. Repositioning CEP-1347, a chemical agent originally developed for the treatment of Parkinson’s disease, as an anti-cancer stem cell drug. Oncotarget. 2017;8:94872–94882. doi: 10.18632/oncotarget.22033. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

