Emerging Importance of Tyrosine Kinase Inhibitors against Cancer: Quo Vadis to Cure?
- PMID: 34769090
- PMCID: PMC8584061
- DOI: 10.3390/ijms222111659
Emerging Importance of Tyrosine Kinase Inhibitors against Cancer: Quo Vadis to Cure?
Abstract
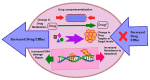
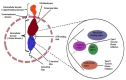
GLOBOCAN 2020 estimated more than 19.3 million new cases, and about 10 million patients were deceased from cancer in 2020. Clinical manifestations showed that several growth factor receptors consisting of transmembrane and cytoplasmic tyrosine kinase (TK) domains play a vital role in cancer progression. Receptor tyrosine kinases (RTKs) are crucial intermediaries of the several cellular pathways and carcinogenesis that directly affect the prognosis and survival of higher tumor grade patients. Tyrosine kinase inhibitors (TKIs) are efficacious drugs for targeted therapy of various cancers. Therefore, RTKs have become a promising therapeutic target to cure cancer. A recent report shows that TKIs are vital mediators of signal transduction and cancer cell proliferation, angiogenesis, and apoptosis. In this review, we discuss the structure and function of RTKs to explore their prime role in cancer therapy. Various TKIs have been developed to date that contribute a lot to treating several types of cancer. These TKI based anticancer drug molecules are also discussed in detail, incorporating their therapeutic efficacy, mechanism of action, and side effects. Additionally, this article focuses on TKIs which are running in the clinical trial and pre-clinical studies. Further, to gain insight into the pathophysiological mechanism of TKIs, we also reviewed the impact of RTK resistance on TKI clinical drugs along with their mechanistic acquired resistance in different cancer types.
Keywords: cancer; clinical trials; drug resistance; mutation; receptor tyrosine kinases; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


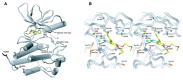
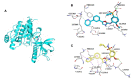
References
-
- Metibemu D.S., Akinloye O.A., Akamo A.J., Ojo D.A., Okeowo O.T., Omotuyi I.O. Exploring receptor tyrosine kinases-inhibitors in cancer treatments. Egypt. J. Med. Hu. Genet. 2019;20 doi: 10.1186/s43042-019-0035-0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

