Chenodeoxycholic Acid Has Non-Thermogenic, Mitodynamic Anti-Obesity Effects in an In Vitro CRISPR/Cas9 Model of Bile Acid Receptor TGR5 Knockdown
- PMID: 34769169
- PMCID: PMC8584144
- DOI: 10.3390/ijms222111738
Chenodeoxycholic Acid Has Non-Thermogenic, Mitodynamic Anti-Obesity Effects in an In Vitro CRISPR/Cas9 Model of Bile Acid Receptor TGR5 Knockdown
Abstract
Bile acids (BA) have shown promising effects in animal models of obesity. However, the said effects are thought to rely on a thermogenic effect, which is questionably present in humans. A previous work has shown that the BA chenodeoxycholic acid (CDCA) can revert obesity and accelerate metabolism in animal and cell culture models. Thus, the aim of this study was to understand if this obesity reduction is indeed thermogenically-dependent. A CRISPR/Cas9 model of TGR5 (BA receptor) knockdown in 3T3-L1 adipocytes was developed to diminish thermogenic effects. Various parameters were assessed, including mitochondrial bioenergetics by Seahorse flux analysis, oxidative stress and membrane potential by fluorometry, intermediary metabolism by NMR, protein content assessment by Western Blot, gene expression by qPCR, and confocal microscopy evaluation of mitophagy. CDCA was still capable, for the most part, of reversing the harmful effects of cellular obesity, elevating mitophagy and leading to the reduction of harmed mitochondria within the cells, boosting mitochondrial activity, and thus energy consumption. In summary, CDCA has a non-thermogenic, obesity reducing capacity that hinges on a healthy mitochondrial population, explaining at least some of these effects and opening avenues of human treatment for metabolic diseases.
Keywords: 3T3-L1; CDCA; CRISPR/Cas9; TGR5; mitochondria; mitophagy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

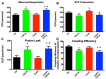

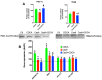
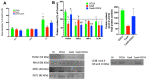

References
-
- Abarca-Gómez L., Abdeen Z.A., Hamid Z.A., Abu-Rmeileh N.M., Acosta-Cazares B., Acuin C., Adams R.J., Aekplakorn W., Afsana K., Aguilar-Salinas C.A., et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- UIDB/04539/2020/Fundação para a Ciência e Tecnologia
- SFRH/BPD/94036/2013/Fundação para a Ciência e Tecnologia
- CEECIND/4400/2017/Fundação para a Ciência e Tecnologia
- HEALTHYAGING 2020 CENTRO-01-0145-FEDER-000012/Fundação para a Ciência e Tecnologia
- PD/BD/114173/2016/Fundação para a Ciência e Tecnologia
LinkOut - more resources
Full Text Sources
Medical

