Periodontitis and Gestational Diabetes Mellitus: A Potential Inflammatory Vicious Cycle
- PMID: 34769262
- PMCID: PMC8584134
- DOI: 10.3390/ijms222111831
Periodontitis and Gestational Diabetes Mellitus: A Potential Inflammatory Vicious Cycle
Abstract
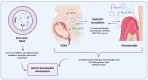
Periodontitis is a chronic inflammatory immune disease associated with a dysbiotic state, influenced by keystone bacterial species responsible for disrupting the periodontal tissue homeostasis. Furthermore, the severity of periodontitis is determined by the interaction between the immune cell response in front of periodontitis-associated species, which leads to the destruction of supporting periodontal tissues and tooth loss in a susceptible host. The persistent bacterial challenge induces modifications in the permeability and ulceration of the sulcular epithelium, which facilitates the systemic translocation of periodontitis-associated bacteria into distant tissues and organs. This stimulates the secretion of pro-inflammatory molecules and a chronic activation of immune cells, contributing to a systemic pro-inflammatory status that has been linked with a higher risk of several systemic diseases, such as type 2 diabetes mellitus (T2DM) and gestational diabetes mellitus (GDM). Although periodontitis and GDM share the common feature of systemic inflammation, the molecular mechanistic link of this association has not been completely clarified. This review aims to examine the potential biological mechanisms involved in the association between periodontitis and GDM, highlighting the contribution of both diseases to systemic inflammation and the role of new molecular participants, such as extracellular vesicles and non-coding RNAs, which could act as novel molecular intercellular linkers between periodontal and placental tissues.
Keywords: extracellular vesicles; gestational diabetes mellitus; periodontitis; systemic inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vos T., Barber R.M., Bell B., Bertozzi-Villa A., Biryukov S., Bolliger I., Charlson F., Davis A., Degenhardt L., Dicker D., et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

